the-sleepy-chemist
60 posts
Latest Posts by the-sleepy-chemist
Roses are red Your titriant is pink You failed Go throw your diploma in the sink
Roses are redYour titrant is pinkYou’re not within 2% of the actual valueYou automatically drop a full letter grade on this assignment because achem is a bitch
Mammals both produce milk and have hair. Ergo, a coconut is a mammal.
nem sirok csak 65ezren belementek a szemembe
A crowd of 65,000 sings ‘Bohemian Rhapsody’ perfectly while waiting for a Green Day concert
“i should drink more water” i remind myself, halfway through my fifth coffee

Straight Outta Answers

Two years ago, a goddamn blue (yes, blue) dress got the world into a tiz over colour perception, and in November last year, it was a pair of blue and black (or are they white and gold?) Havaianas that divided families and friendships worldwide.
Now a Japanese psychologist has created another mind-warping colour illusion by swapping the red pixels for grey ones in a photo of a strawberry tart, demonstrating once again that our brains have the final say on colour.
Akiyoshi Kitaoka from Ritsumeikan University in Japan stirred up a storm recently by tweeting a filtered photo of a strawberry tart and announcing there were no red pixels in the picture, contrary to what it might look like.
Continue Reading.

Nothing can stop us!
Largest Batch of Earth-size, Habitable Zone Planets
Our Spitzer Space Telescope has revealed the first known system of seven Earth-size planets around a single star. Three of these planets are firmly located in an area called the habitable zone, where liquid water is most likely to exist on a rocky planet.

This exoplanet system is called TRAPPIST-1, named for The Transiting Planets and Planetesimals Small Telescope (TRAPPIST) in Chile. In May 2016, researchers using TRAPPIST announced they had discovered three planets in the system.

Assisted by several ground-based telescopes, Spitzer confirmed the existence of two of these planets and discovered five additional ones, increasing the number of known planets in the system to seven.

This is the FIRST time three terrestrial planets have been found in the habitable zone of a star, and this is the FIRST time we have been able to measure both the masses and the radius for habitable zone Earth-sized planets.
All of these seven planets could have liquid water, key to life as we know it, under the right atmospheric conditions, but the chances are highest with the three in the habitable zone.

At about 40 light-years (235 trillion miles) from Earth, the system of planets is relatively close to us, in the constellation Aquarius. Because they are located outside of our solar system, these planets are scientifically known as exoplanets. To clarify, exoplanets are planets outside our solar system that orbit a sun-like star.

In this animation, you can see the planets orbiting the star, with the green area representing the famous habitable zone, defined as the range of distance to the star for which an Earth-like planet is the most likely to harbor abundant liquid water on its surface. Planets e, f and g fall in the habitable zone of the star.
Using Spitzer data, the team precisely measured the sizes of the seven planets and developed first estimates of the masses of six of them. The mass of the seventh and farthest exoplanet has not yet been estimated.

For comparison…if our sun was the size of a basketball, the TRAPPIST-1 star would be the size of a golf ball.
Based on their densities, all of the TRAPPIST-1 planets are likely to be rocky. Further observations will not only help determine whether they are rich in water, but also possibly reveal whether any could have liquid water on their surfaces.
The sun at the center of this system is classified as an ultra-cool dwarf and is so cool that liquid water could survive on planets orbiting very close to it, closer than is possible on planets in our solar system. All seven of the TRAPPIST-1 planetary orbits are closer to their host star than Mercury is to our sun.

The planets also are very close to each other. How close? Well, if a person was standing on one of the planet’s surface, they could gaze up and potentially see geological features or clouds of neighboring worlds, which would sometimes appear larger than the moon in Earth’s sky.

The planets may also be tidally-locked to their star, which means the same side of the planet is always facing the star, therefore each side is either perpetual day or night. This could mean they have weather patterns totally unlike those on Earth, such as strong wind blowing from the day side to the night side, and extreme temperature changes.

Because most TRAPPIST-1 planets are likely to be rocky, and they are very close to one another, scientists view the Galilean moons of Jupiter – lo, Europa, Callisto, Ganymede – as good comparisons in our solar system. All of these moons are also tidally locked to Jupiter. The TRAPPIST-1 star is only slightly wider than Jupiter, yet much warmer.
How Did the Spitzer Space Telescope Detect this System?
Spitzer, an infrared telescope that trails Earth as it orbits the sun, was well-suited for studying TRAPPIST-1 because the star glows brightest in infrared light, whose wavelengths are longer than the eye can see. Spitzer is uniquely positioned in its orbit to observe enough crossing (aka transits) of the planets in front of the host star to reveal the complex architecture of the system.

Every time a planet passes by, or transits, a star, it blocks out some light. Spitzer measured the dips in light and based on how big the dip, you can determine the size of the planet. The timing of the transits tells you how long it takes for the planet to orbit the star.

The TRAPPIST-1 system provides one of the best opportunities in the next decade to study the atmospheres around Earth-size planets. Spitzer, Hubble and Kepler will help astronomers plan for follow-up studies using our upcoming James Webb Space Telescope, launching in 2018. With much greater sensitivity, Webb will be able to detect the chemical fingerprints of water, methane, oxygen, ozone and other components of a planet’s atmosphere.
At 40 light-years away, humans won’t be visiting this system in person anytime soon…that said…this poster can help us imagine what it would be like:

Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com

Not just one, but seven Earth-size planets that could potentially harbor life have been identified orbiting a tiny star not too far away, offering the first realistic opportunity to search for signs of alien life outside of the solar system.
The planets orbit a dwarf star named Trappist-1, about 40 light years, or some 235 trillion miles, from Earth. That is quite close in cosmic terms, and by happy accident, the orientation of the orbits of the seven planets allows them to be studied in great detail.
One or more of the exoplanets in this new system could be at the right temperature to be awash in oceans of water, astronomers said, based on the distance of the planets from the dwarf star.
“This is the first time so many planets of this kind are found around the same star,” said Michael Gillon, an astronomer at the University of Liege in Belgium and the leader of an international team that has been observing Trappist-1.
Continue Reading.
maybe if im under enough blankets, the Responsibilities wont be able to find me
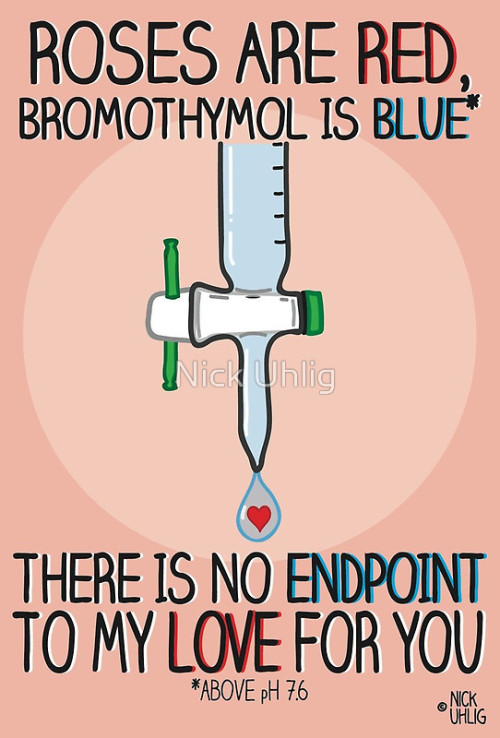
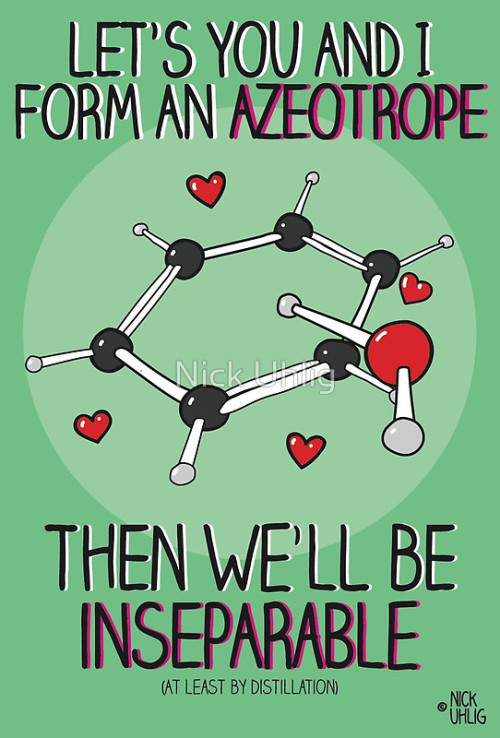
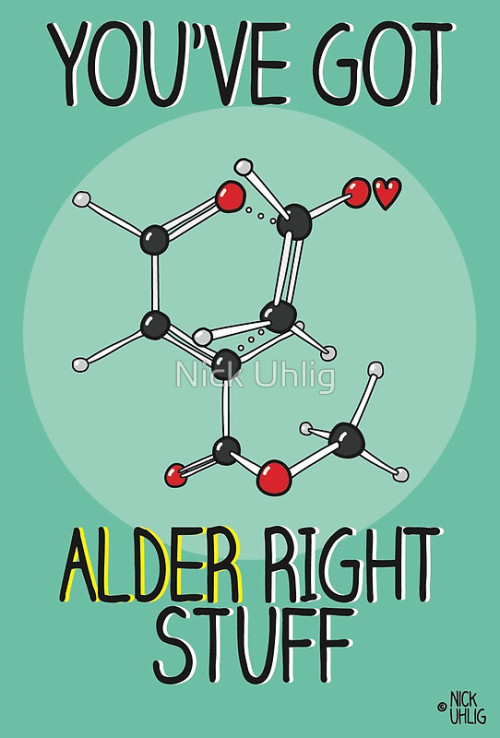
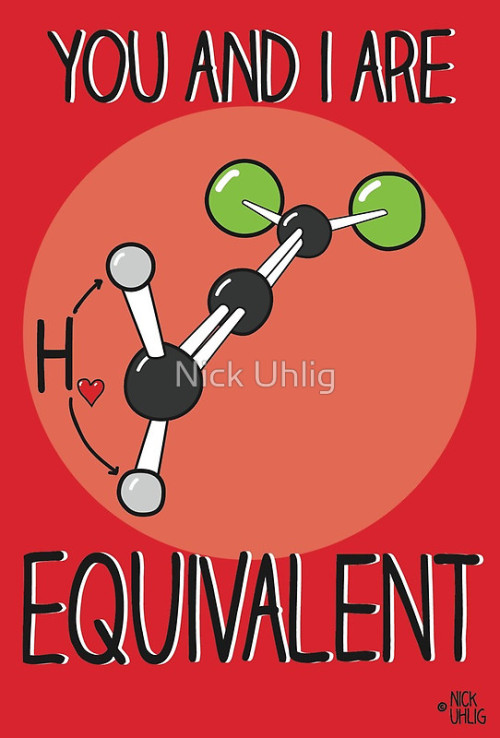


Chemistry Valentine’s Cards by Nick Uhlig.
And now, we shall pretend to be mathematicians.
Quantum mechanics lecturer on solving the Hermite equation (via mathprofessorquotes)
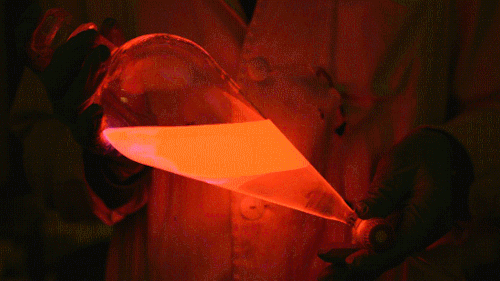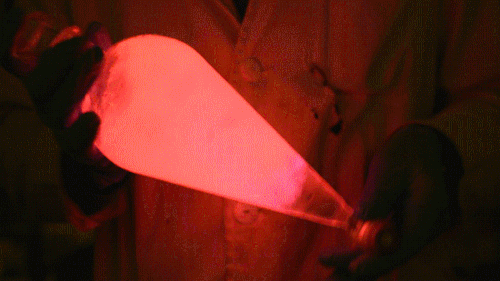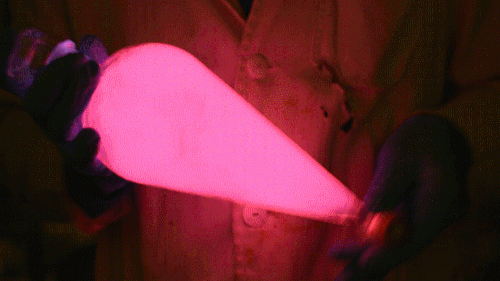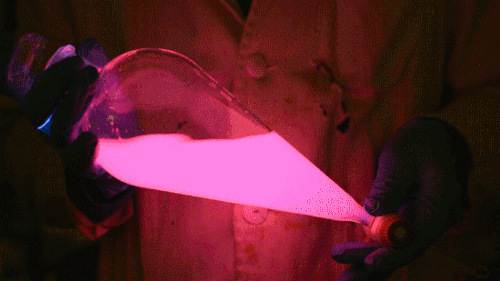
My favorite thing from this year: playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye.
Using Nile Red:
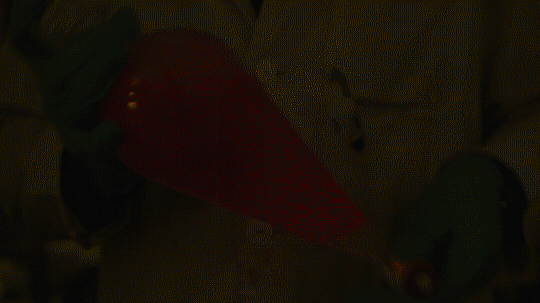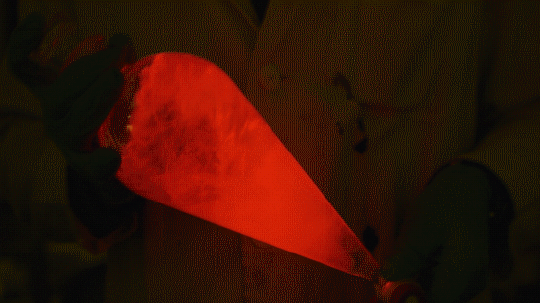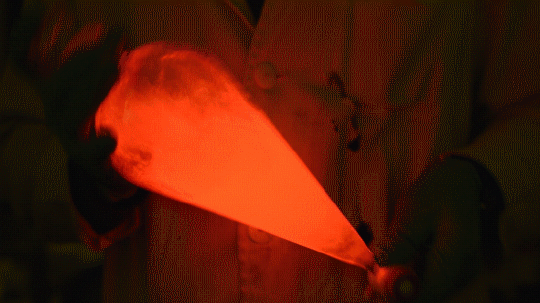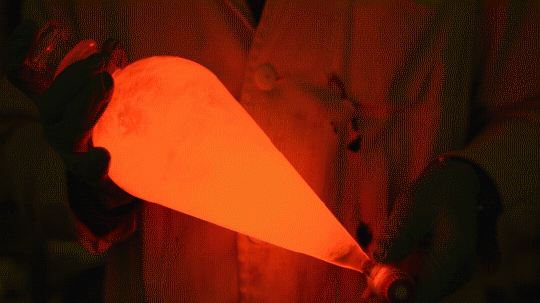
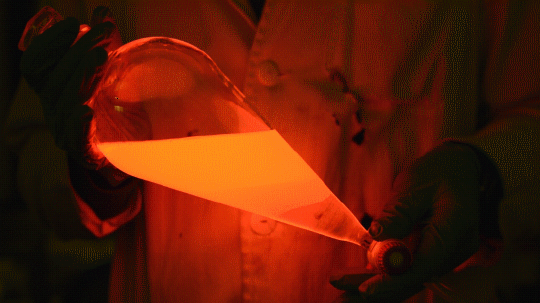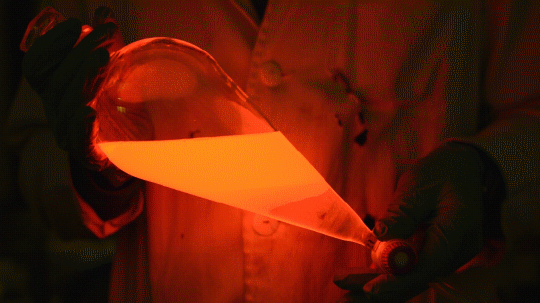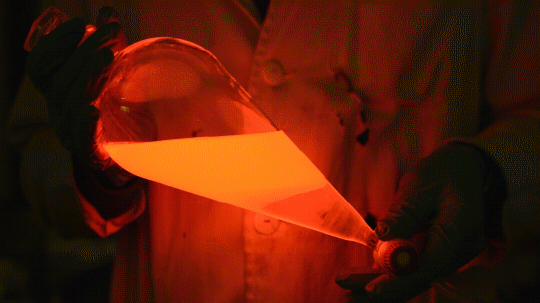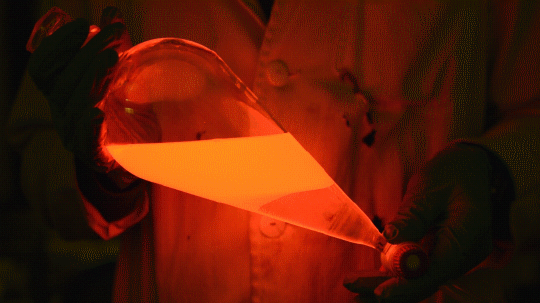
Mixing it with Perylene:


During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on the video.
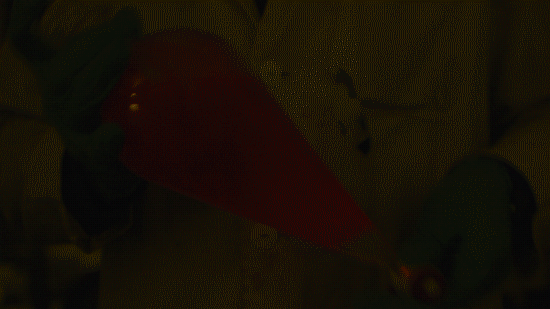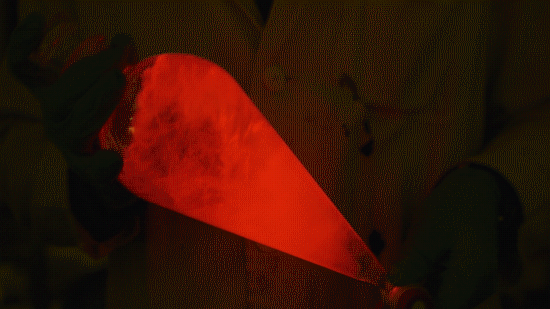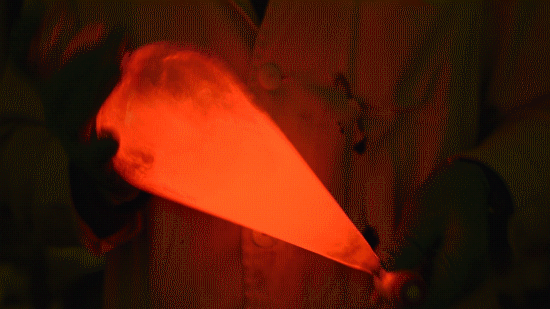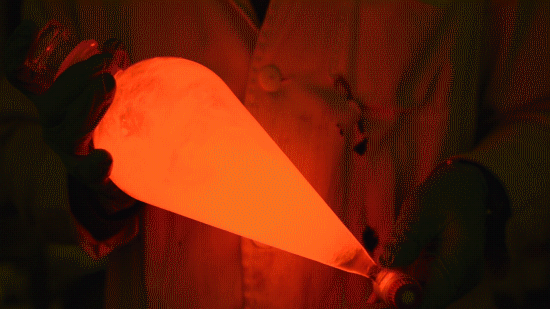
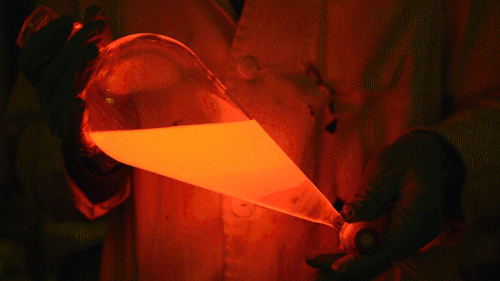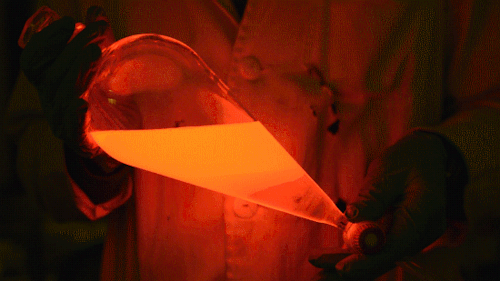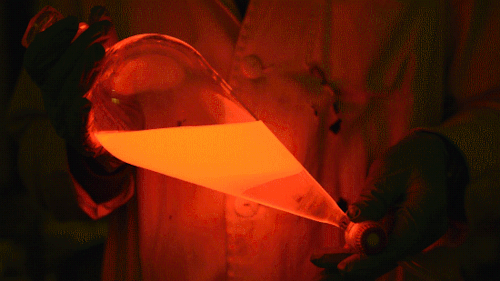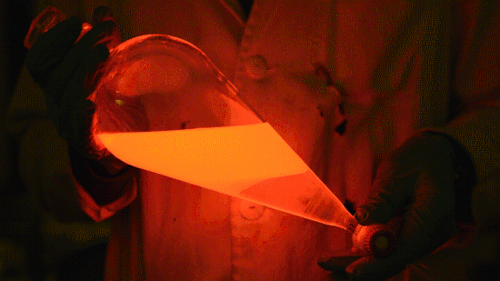

Playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye. The chemistry behind the reaction is something like this:

During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on this video:
Lab life
Constantly using acetone to wash your glassware which ends up making your nails super brittle 💔

On military live fire training ranges, troops practice firing artillery shells, drop bombs on old tanks or derelict buildings and test the capacity of new weapons.
But those explosives and munitions leave behind toxic compounds that have contaminated millions of acres of U.S. military bases – with an estimated cleanup bill ranging between $16 billion and $165 billion.
In a paper published online Nov. 16 in Plant Biotechnology Journal, University of Washington and University of York researchers describe new transgenic grass species that can neutralize and eradicate RDX – a toxic compound that has been widely used in explosives since World War II.
UW engineers introduced two genes from bacteria that learned to eat RDX and break it down into harmless components in two perennial grass species: switchgrass (Panicum virgatum) and creeping bentgrass (Agrostis stolonifera). The best-performing strains removed all the RDX from a simulated soil in which they were grown within less than two weeks, and they retained none of the toxic chemical in their leaves or stems.
Continue Reading.

This week is Antibiotic Awareness Week – learn more about the different types of antibiotics with this graphic!
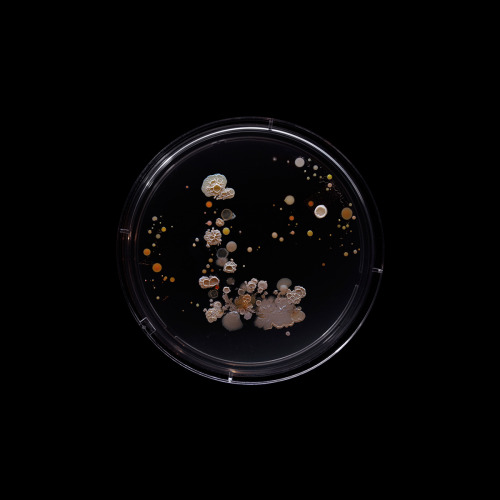

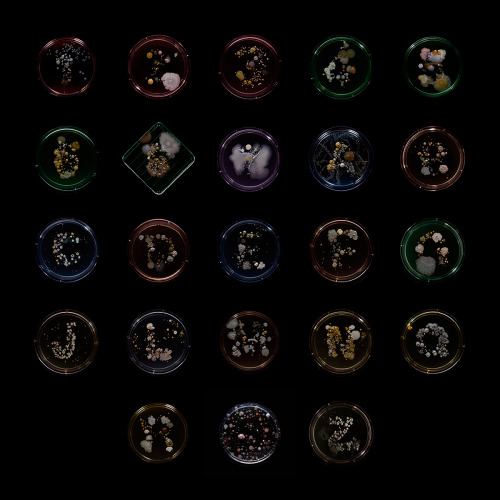
Subvisual Subway - Bacteria of the New York City subway- Craig Ward

COFFEE STAIN UNDER A MICROSCOPE
Vin Kitayama, an artist and environmental researcher, created this image from something fairly mundane: an evaporating drop of espresso. Kitayama placed the drop on a microscope slide and then snapped pictures through a polarized light microscope at 4× magnification. As the coffee dried, solid compounds that were dissolved in the coffee, such as caffeine and chlorogenic acid, started forming small crystals. In the polarized light, these crystals shimmered different colors. The image won 9th place in the Nikon Small World photomicrography competition.
Credit: Vin Kitayama
More Chemistry in Pictures and C&EN content:
U.S. Senators Push for Ban on Caffeine Powder
Caffeinated Cocrystals
Tweaking Coffee’s Flavor Chemistry

KISS OF DEATH
One of the reasons cancer is so hard to defeat is that the body’s immune system has trouble recognizing cancer cells growing among healthy cells. Some scientists want to help. Researchers designed mouse T cells to specifically bind to a protein complex on fibrosarcoma MC57 tumor cell membranes. In this sped-up video, once the T cells (each about 10 μm across) meet their targets, they create holes in the cancer cell membranes using a protein called perforin. Next, the immune cells flood the pierced cells with a rush of cell-killing granules called granzymes. Propidium iodide, a dye the scientists added to the plate of cells, also squeezes in through the hole and starts glowing red when it comes in contact with RNA and DNA inside the cancer cells. This tells the researchers that the cells have been pierced and will soon die. This process takes about 75 minutes in real time.
Credit: Misty Jenkins (Read the paper.)
Related C&EN content:
The Immune System Fights Back
Cancer-Killing Machine






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto

A 25-year-old student has just come up with a way to fight drug-resistant superbugs without antibiotics.
The new approach has so far only been tested in the lab and on mice, but it could offer a potential solution to antibiotic resistance, which is now getting so bad that the United Nations recently declared it a “fundamental threat” to global health.
Antibiotic-resistant bacteria already kill around 700,000 people each year, but a recent study suggests that number could rise to around 10 million by 2050.
In addition to common hospital superbug, methicillin-resistant Staphylococcus aureus (MRSA), scientists are now also concerned that gonorrhoea is about tobecome resistant to all remaining drugs.
But Shu Lam, a 25-year-old PhD student at the University of Melbourne in Australia, has developed a star-shaped polymer that can kill six different superbug strains without antibiotics, simply by ripping apart their cell walls.
“We’ve discovered that [the polymers] actually target the bacteria and kill it in multiple ways,” Lam told Nicola Smith from The Telegraph. “One method is by physically disrupting or breaking apart the cell wall of the bacteria. This creates a lot of stress on the bacteria and causes it to start killing itself.”
The research has been published in Nature Microbiology, and according to Smith, it’s already being hailed by scientists in the field as “a breakthrough that could change the face of modern medicine”.
Before we get too carried away, it’s still very early days. So far, Lam has only tested her star-shaped polymers on six strains of drug-resistant bacteria in the lab, and on one superbug in live mice.
But in all experiments, they’ve been able to kill their targeted bacteria - and generation after generation don’t seem to develop resistance to the polymers.
Continue Reading.
Using the Power of Space to Fight Cancer
From cancer research to DNA sequencing, the International Space Space is proving to be an ideal platform for medical research. But new techniques in fighting cancer are not confined to research on the space station. Increasingly, artificial intelligence is helping to “read” large datasets. And for the past 15 years, these big data techniques pioneered by our Jet Propulsion Laboratory have been revolutionizing biomedical research.
Microgravity Research on Space Station
On Earth, scientists have devised several laboratory methods to mimic normal cellular behavior, but none of them work exactly the way the body does. Beginning more than 40 years ago aboard Skylab and continuing today aboard the space station, we and our partners have conducted research in the microgravity of space. In this environment, in vitro cells arrange themselves into three-dimensional groupings, or aggregates. These aggregates more closely resemble what actually occurs in the human body. Cells in microgravity also tend to clump together more easily, and they experience reduced fluid shear stress – a type of turbulence that can affect their behavior. The development of 3D structure and enhanced cell differentiation seen in microgravity may help scientists study cell behavior and cancer development in models that behave more like tissues in the human body.

In addition, using the distinctive microgravity environment aboard the station, researchers are making further advancements in cancer therapy. The process of microencapsulation was investigated aboard the space station in an effort to improve the Earth-based technology. Microencapsulation is a technique that creates tiny, liquid-filled, biodegradable micro-balloons that can serve as delivery systems for various compounds, including specific combinations of concentrated anti-tumor drugs. For decades, scientists and clinicians have looked for the best ways to deliver these micro-balloons, or microcapsules, directly to specific treatment sites within a cancer patient, a process that has the potential to revolutionize cancer treatment.

A team of scientists at Johnson Space Center used the station as a tool to advance an Earth-based microencapsulation system, known as the Microencapsulation Electrostatic Processing System-II (MEPS-II), as a way to make more effective microcapsules. The team leveraged fluid behavior in microgravity to develop a new technique for making these microcapsules that would be more effective on Earth. In space, microgravity brought together two liquids incapable of mixing on Earth (80 percent water and 20 percent oil) in such a way that spontaneously caused liquid-filled microcapsules to form as spherical, tiny, liquid-filled bubbles surrounded by a thin, semipermeable, outer membrane. After studying these microcapsules on Earth, the team was able to develop a system to make more of the space-like microcapsules on Earth and are now performing activities leading to FDA approval for use in cancer treatment.

In addition, the ISS National Laboratory managed by the Center for the Advancement of Science in Space (CASIS) has also sponsored cancer-related investigations. An example of that is an investigation conducted by the commercial company Eli Lilly that seeks to crystallize a human membrane protein involved in several types of cancer together with a compound that could serve as a drug to treat those cancers.
“So many things change in 3-D, it’s mind-blowing – when you look at the function of the cell, how they present their proteins, how they activate genes, how they interact with other cells,” said Jeanne Becker, Ph.D., a cell biologist at Nano3D Biosciences in Houston and principal investigator for a study called Cellular Biotechnology Operations Support Systems: Evaluation of Ovarian Tumor Cell Growth and Gene Expression, also known as the CBOSS-1-Ovarian study. “The variable that you are most looking at here is gravity, and you can’t really take away gravity on Earth. You have to go where gravity is reduced."
Crunching Big Data Using Space Knowledge

Our Jet Propulsion Laboratory often deals with measurements from a variety of sensors – say, cameras and mass spectrometers that are on our spacecraft. Both can be used to study a star, planet or similar target object. But it takes special software to recognize that readings from very different instruments relate to one another.
There’s a similar problem in cancer research, where readings from different biomedical tests or instruments require correlation with one another. For that to happen, data have to be standardized, and algorithms must be “taught” to know what they’re looking for.
Because space exploration and cancer research share a similar challenge in that they both must analyze large datasets to find meaning, JPL and the National Cancer Institute renewed their research partnership to continue developing methods in data science that originated in space exploration and are now supporting new cancer discoveries.
JPL’s methods are leading to the development of a single, searchable network of cancer data that researcher can work into techniques for the early diagnosis of cancer or cancer risk. In the time they’ve worked together, the two organizations’ efforts have led to the discovery of six new Food and Drug Administration-approved cancer biomarkers. These agency-approved biomarkers have been used in more than 1 million patient diagnostic tests worldwide.
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com
Why Sequencing DNA in Space is a Big Deal
… And How You Can Talk to the Scientists Who Made It Happen

Less than one month ago, DNA had never been sequenced in space. As of today, more than one billion base pairs of DNA have been sequenced aboard the International Space Station, Earth’s only orbiting laboratory. The ability to sequence the DNA of living organisms in space opens a whole new world of scientific and medical possibilities. Scientists consider it a game changer.

NASA astronaut Kate Rubins, who has a background in genomics, conducted the sequencing on the space station as part of the Biomolecule Sequencer investigation. A small, commercial, off-the-shelf device called MinION (min-EYE-ON), manufactured by Oxford Nanopore Technologies in the UK, was used to sequence the DNA of bacteria, a virus and rodents. Human DNA was not sequenced, and there are no immediate plans to sequence human DNA in space.

(Image Credit: Oxford Nanopore Technologies)
The MinION is about the size of a candy bar, and plugs into a laptop or tablet via USB connection, which also provides power to the device. The tiny, plug and play sequencer is diminutive compared to the large microwave-sized sequencers used on Earth, and uses much less power. Unlike other terrestrial instruments whose sequencing run times can take days, this device’s data is available in near real time; analysis can begin within 10-15 minutes from the application of the sample.

Having real-time analysis capabilities aboard the space station could allow crews to identify microbes, diagnose infectious disease and collect genomic and genetic data concerning crew health, without having to wait long periods of time to return samples to Earth and await ground-based analysis.
The first DNA sequencing was conducted on Aug. 26, and on Sept. 14, Rubins and the team of scientists back at NASA’s Johnson Space Center in Houston hit the one-billionth-base-pairs-of-DNA-sequenced mark.

Have more questions about how the Biomolecule Sequencer works, or how it could benefit Earth or further space exploration? Ask the team of scientists behind the investigation, who will be available for questions during a Reddit Ask Me Anything on /r/science on Wednesday, Sept. 29 at 2 p.m. EDT.
The participants are:
Dr. Aaron Burton, NASA Johnson Space Center, Planetary Scientist and Principal Investigator
Dr. Sarah Castro-Wallace, NASA Johnson Space Center, Microbiologist and Project Manager
Dr. David J. Smith, NASA Ames Research Center, Microbiologist
Dr. Mark Lupisella, NASA Goddard Space Flight Center, Systems Engineer
Dr. Jason P. Dworkin, NASA Goddard Space Flight Center, Astrobiologist
Dr. Christopher E. Mason, Weill Cornell Medicine Dept. of Physiology and Biophysics, Associate Professor




Next week I’ll give a presentation on the Researchers Night at Eötvös Loránd University, Hungary with the title: “Chemistry of light and the light of chemistry”.
During this presentation one of my favorite dyes will be also presented: Nile Red. However, just as usual, the 1000 USD/gram price was a bit over our budget, so I had to make it.
The raw product was contaminated with a few impurities, but a fast purification, by simple filtering the mixture through a short column helped a lot and ended up with a +95% pure product.
At first I concentrated the product from a dilute solution on the column as seen on the first pics. It’s interesting to see, that it has a different fluorescence in solution (faint orange fluorescent) and while it’s absorbed on the solid phase (pink, highly fluorescent).
After all the product was on the solid phase, I added another solvent and washed down the pure, HIGHLY FLUORESCENT product. Everything else, what was mainly products of side reactions, stuck at the top of the column as seen on the second pics and the gifs.
Also here is a video from the whole process in HD: https://youtu.be/W0Lk5jkd_B0

This Week in Chemistry: Treating cataracts, LED preservatives, nitrogen glaciers, and more! http://goo.gl/56bcZu


This is a Sea Sapphire! And when it doesn’t look amazing it’s invisible!
This is a type of crustacean called a copepod. It’s back is covered in guanine crystals. If it weren’t for these crystals the Sea Sapphire would be transparent, but these crystals are spaced in such a way that they strongly reflect certain colours of light. The colour of the light that’s reflected is dependent on the angle that it comes in.
Usually, it reflects blue light, but when the light hits the Sea Sapphire at 45 degrees, the reflected light shifts into the ultraviolet. And since we can’t see that it becomes invisible!

Blood Vessels, part 1 - Form and Function By CrashCourse
Now that we’ve discussed blood, we’re beginning our look at how it gets around your body. Today Hank explains your blood vessels and their basic three-layer structure of your blood vessels. We’re also going over how those structures differ slightly in different types of vessels. We will also follow the flow of blood from your heart to capillaries in your right thumb, and all the way back to your heart again. Table of Contents The Basice Three-Layer Structure of Your Blood Vessels 2:17 Different Types of Vessels 3:36 The Flow of Blood From Your Heart to Capillaries 3:59 The Flow From Capillaries to the Heart 7:01
How Smells Trigger Memories By SciShow
SciShow explains how smells can bring back early memories – even memories that your brain didn’t know you had.
Hosted by: Hank Green