Lab Life
Lab life
Constantly using acetone to wash your glassware which ends up making your nails super brittle 💔
More Posts from The-sleepy-chemist and Others
nem sirok csak 65ezren belementek a szemembe
A crowd of 65,000 sings ‘Bohemian Rhapsody’ perfectly while waiting for a Green Day concert




Feathertail Centipedes are an entire genus of centipedes from eastern Africa.
Their hindmost legs are amazing, bizarre and rather beautiful. They’re extremely long, often brightly coloured and always flattened into strange feather shapes.
The centipede uses these crazy legs to warn predators and other interfering beasts of their venomous presence.
Waving the legs from side to side causes them to emit a kind of rustling sound as one patch of leg rubs up against another patch of the same leg. It’s sort of like how crickets sing by rubbing their wings together.
They don’t, however, rub one leg against the other. Each leg makes the noise on its own… in more ways than one. The centipede can lop off one of its special legs and it will keep waving around making noise. Hopefully it will prove distracting enough that the Feathertail can make an escape.
It makes you wonder… how long before we get flying centipedes?
…Images: Frupus

Aurora - Nutirwik Creek, Brooks Range, Alaska | by Fred Wasmer






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto

Bobbit Worm. By: ken & anita’s photos
The Bobbit worm, Eunice aphroditois, is a ferocious underwater predator. Armed with sharp teeth, it is known to attack with such speeds that its prey is sometimes sliced in half. Though they do vary in size, they have been recorded to grow up to nine feet tall.
And now, we shall pretend to be mathematicians.
Quantum mechanics lecturer on solving the Hermite equation (via mathprofessorquotes)
Science never goes out of STYLE! And we prove it with this scientific a capella cover song!
Submitted by asapscience.
Submit some science here! :)
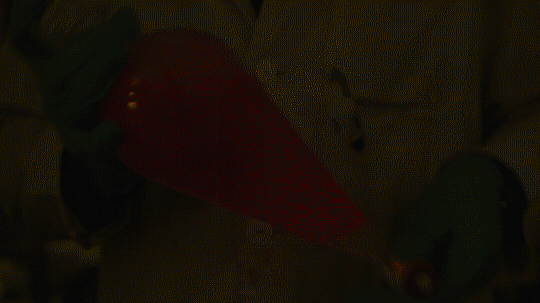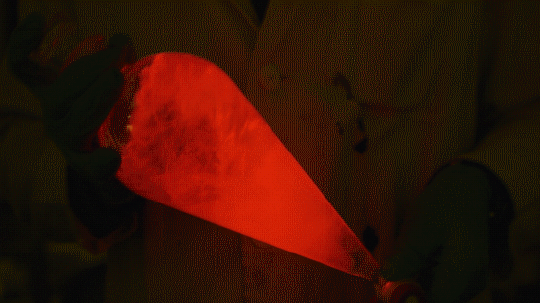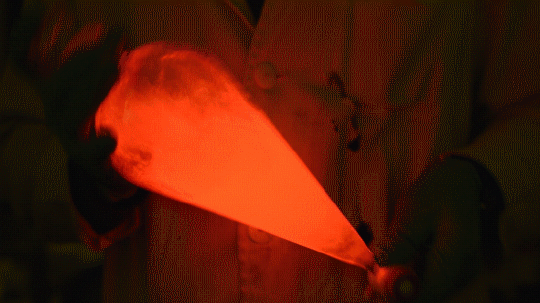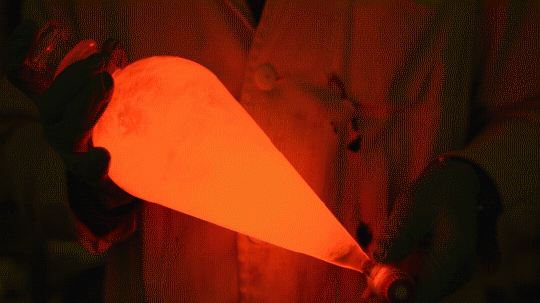
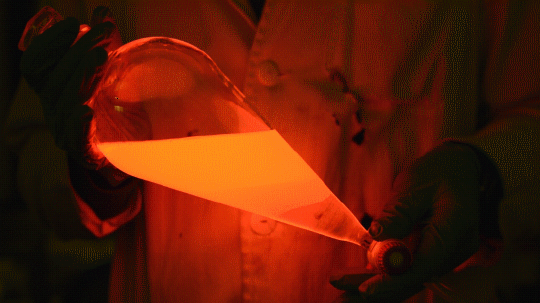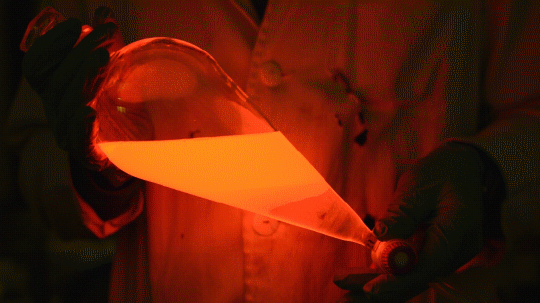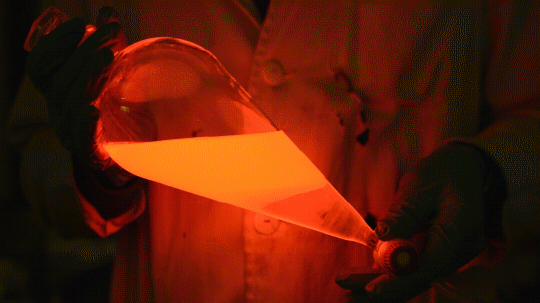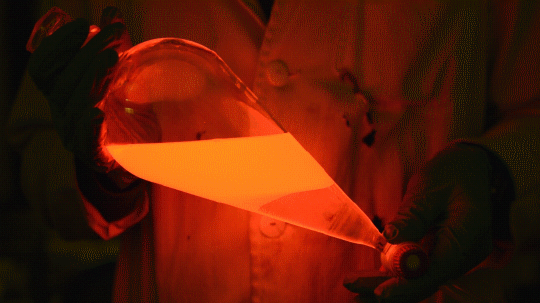
My favorite thing from this year: playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye.
Using Nile Red:
Mixing it with Perylene:


During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on the video.
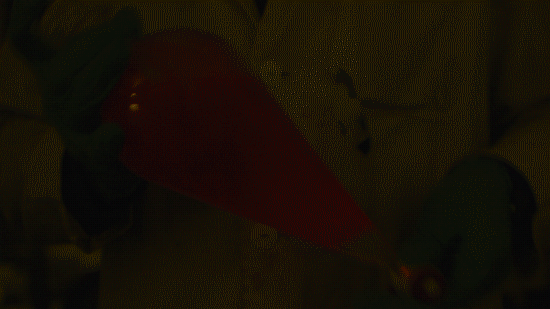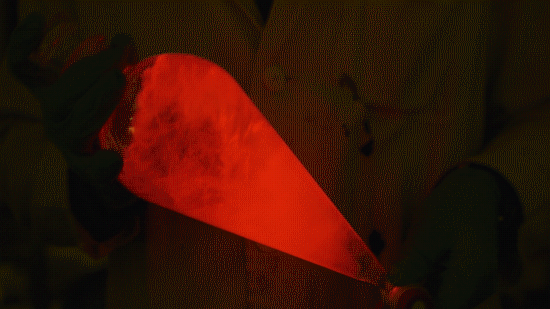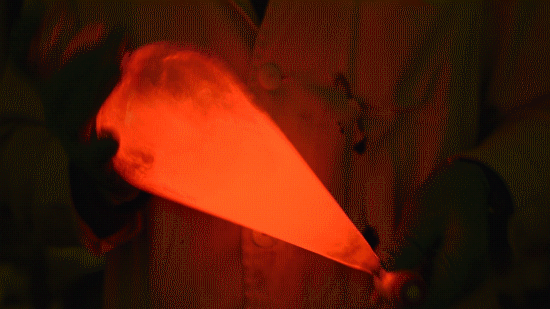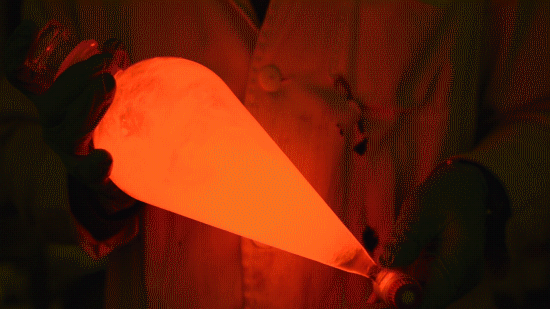
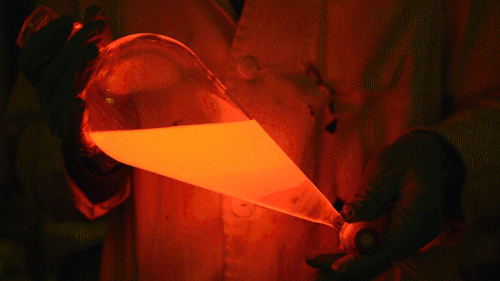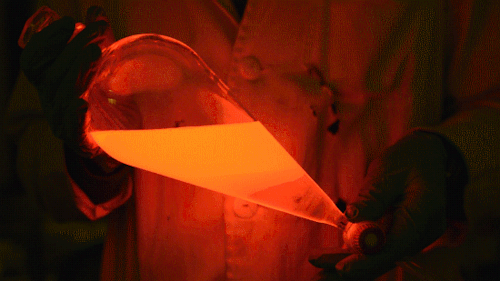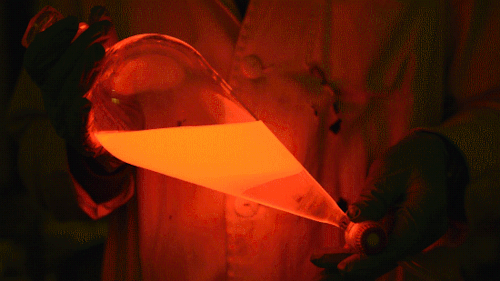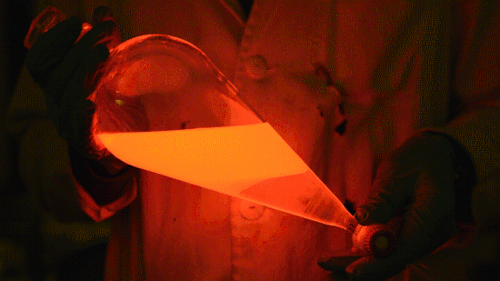
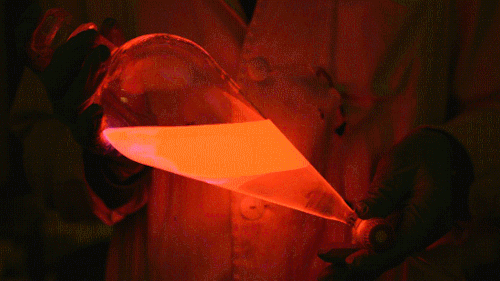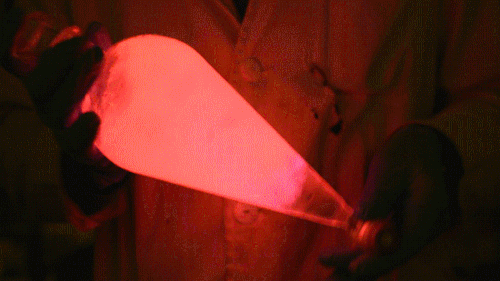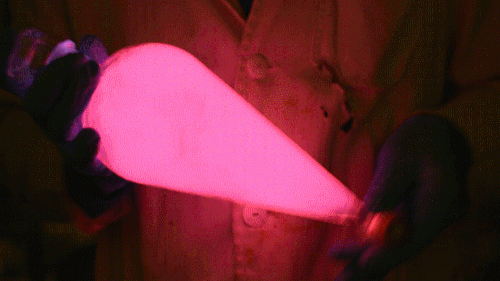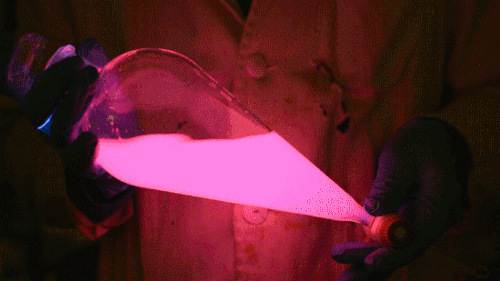

Playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye. The chemistry behind the reaction is something like this:

During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on this video:
How Smells Trigger Memories By SciShow
SciShow explains how smells can bring back early memories – even memories that your brain didn’t know you had.
Hosted by: Hank Green
-
 besserlebenmitchemie liked this · 5 years ago
besserlebenmitchemie liked this · 5 years ago -
 monicakarem liked this · 7 years ago
monicakarem liked this · 7 years ago -
 atrosan liked this · 7 years ago
atrosan liked this · 7 years ago -
 stephengordon liked this · 7 years ago
stephengordon liked this · 7 years ago -
 bulacan011 liked this · 8 years ago
bulacan011 liked this · 8 years ago -
 doomed-cinnamonroll liked this · 8 years ago
doomed-cinnamonroll liked this · 8 years ago -
 awesomenewchurch-blog liked this · 8 years ago
awesomenewchurch-blog liked this · 8 years ago -
 missprettykatty liked this · 8 years ago
missprettykatty liked this · 8 years ago -
 auroramere liked this · 8 years ago
auroramere liked this · 8 years ago -
 jestersditty liked this · 8 years ago
jestersditty liked this · 8 years ago -
 spirit-of-science reblogged this · 8 years ago
spirit-of-science reblogged this · 8 years ago -
 my-bobohu-blog liked this · 8 years ago
my-bobohu-blog liked this · 8 years ago -
 musthavebeenhigh liked this · 8 years ago
musthavebeenhigh liked this · 8 years ago -
 uss-birdbrain liked this · 8 years ago
uss-birdbrain liked this · 8 years ago -
 lusciouslesbean liked this · 8 years ago
lusciouslesbean liked this · 8 years ago -
 diddlycapucccinoo-blog liked this · 8 years ago
diddlycapucccinoo-blog liked this · 8 years ago -
 dagiovana-blog liked this · 8 years ago
dagiovana-blog liked this · 8 years ago -
 whatislife5603-blog liked this · 8 years ago
whatislife5603-blog liked this · 8 years ago -
 chaosanna liked this · 8 years ago
chaosanna liked this · 8 years ago -
 ksjdkajwi liked this · 8 years ago
ksjdkajwi liked this · 8 years ago -
 caitice-blog liked this · 8 years ago
caitice-blog liked this · 8 years ago -
 iamthinking liked this · 8 years ago
iamthinking liked this · 8 years ago -
 violetbeetles-blog reblogged this · 8 years ago
violetbeetles-blog reblogged this · 8 years ago -
 melanierxse liked this · 8 years ago
melanierxse liked this · 8 years ago -
 ajoanp-blog liked this · 8 years ago
ajoanp-blog liked this · 8 years ago -
 teredo-navalis liked this · 8 years ago
teredo-navalis liked this · 8 years ago -
 pleasantlyuniversallyunknown liked this · 8 years ago
pleasantlyuniversallyunknown liked this · 8 years ago -
 picasdan liked this · 8 years ago
picasdan liked this · 8 years ago -
 xytoskeleton reblogged this · 8 years ago
xytoskeleton reblogged this · 8 years ago -
 zvezdydusha liked this · 8 years ago
zvezdydusha liked this · 8 years ago -
 the-sleepy-chemist liked this · 8 years ago
the-sleepy-chemist liked this · 8 years ago -
 the-sleepy-chemist reblogged this · 8 years ago
the-sleepy-chemist reblogged this · 8 years ago -
 aye-aronn liked this · 8 years ago
aye-aronn liked this · 8 years ago -
 dafuqisft liked this · 8 years ago
dafuqisft liked this · 8 years ago -
 briceskater reblogged this · 8 years ago
briceskater reblogged this · 8 years ago -
 mastarija liked this · 8 years ago
mastarija liked this · 8 years ago -
 mansgottohaveacode liked this · 8 years ago
mansgottohaveacode liked this · 8 years ago -
 lookatdatmoooon-blog liked this · 8 years ago
lookatdatmoooon-blog liked this · 8 years ago -
 fangul liked this · 8 years ago
fangul liked this · 8 years ago -
 occhinelcielo liked this · 8 years ago
occhinelcielo liked this · 8 years ago -
 studyingchemistry reblogged this · 8 years ago
studyingchemistry reblogged this · 8 years ago
60 posts