How Smells Trigger Memories By SciShow
How Smells Trigger Memories By SciShow
SciShow explains how smells can bring back early memories – even memories that your brain didn’t know you had.
Hosted by: Hank Green
More Posts from The-sleepy-chemist and Others






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto


The Tsars’s vodka in action. Aqua regia or Царская водка in Russian is a 3/1 mixture of hydrochloric acid and nitric acid.
Upon mixing concentrated hydrochloric acid and concentrated nitric acid a chemical reactions occurs. The product of the reaction is nitrosyl chloride and chlorine as evidenced by the fuming nature and characteristic yellow color of aqua regia. In this case the dissolved copper and other transition metals turned the color of the solution deep green, but the gas over the solution is yellow from the chlorine and nitrous fumes.
Interesting fact about the Nobel prize and the dissolution of gold:
When Nazi Germany occupied Denmark from April 1940, during World War II, György de Hevesy dissolved the gold Nobel Prizes of Max von Laue and James Franck with aqua regia; it was illegal at the time to send gold out of the country, and were it discovered that Laue and Franck had done so to prevent them from being stolen, they could have faced prosecution in Germany. He placed the resulting solution on a shelf in his laboratory at the Niels Bohr Institute. After the war, he returned to find the solution undisturbed and precipitated the gold out of the acid. The Nobel Society then recast the Nobel Prizes using the original gold.
George de Hevesy got his Noble Prize in Chemistry for ”for his work on the use of isotopes as tracers in the study of chemical processes” in 1943.
Max von Laue got his Nobel Prize in Physics for ”for his discovery of the diffraction of X-rays by crystals” in 1914.
James Franck got his Nobel Prize in Physics ”for his discovery of the laws governing the impact of an electron upon an atom” in 1925.

Not just one, but seven Earth-size planets that could potentially harbor life have been identified orbiting a tiny star not too far away, offering the first realistic opportunity to search for signs of alien life outside of the solar system.
The planets orbit a dwarf star named Trappist-1, about 40 light years, or some 235 trillion miles, from Earth. That is quite close in cosmic terms, and by happy accident, the orientation of the orbits of the seven planets allows them to be studied in great detail.
One or more of the exoplanets in this new system could be at the right temperature to be awash in oceans of water, astronomers said, based on the distance of the planets from the dwarf star.
“This is the first time so many planets of this kind are found around the same star,” said Michael Gillon, an astronomer at the University of Liege in Belgium and the leader of an international team that has been observing Trappist-1.
Continue Reading.
Lab life
Constantly using acetone to wash your glassware which ends up making your nails super brittle 💔
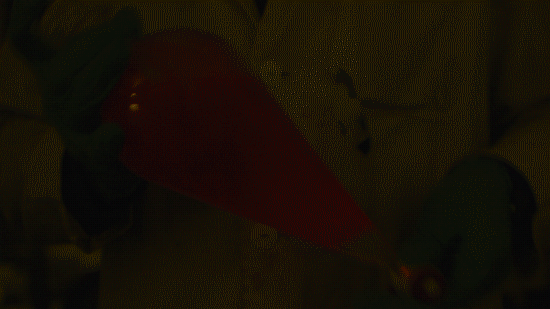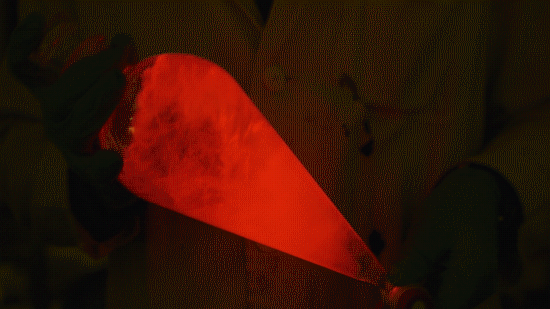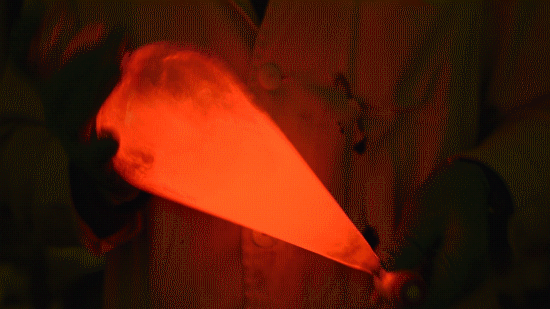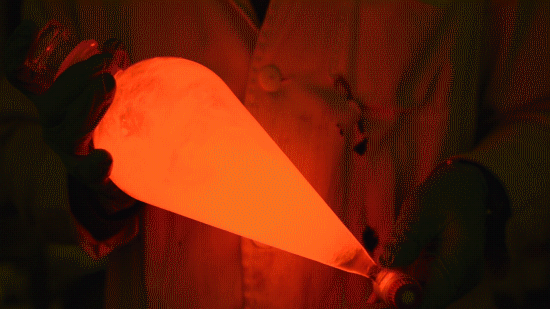
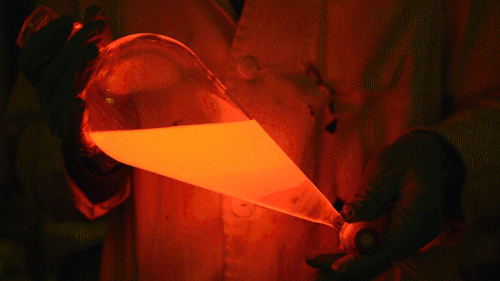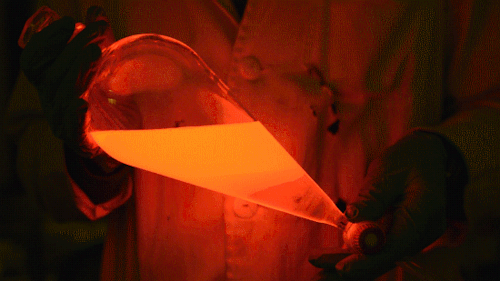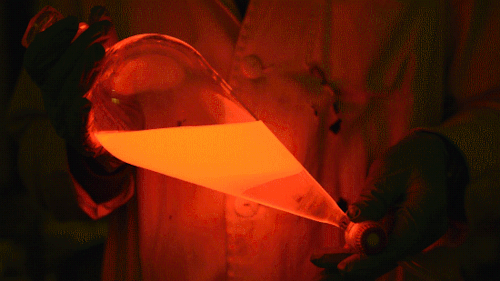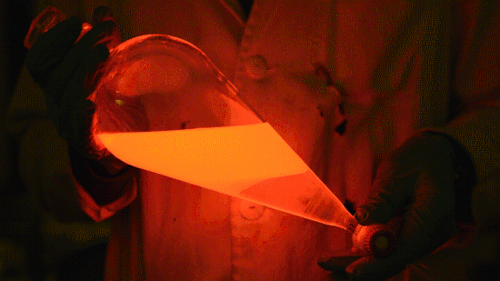
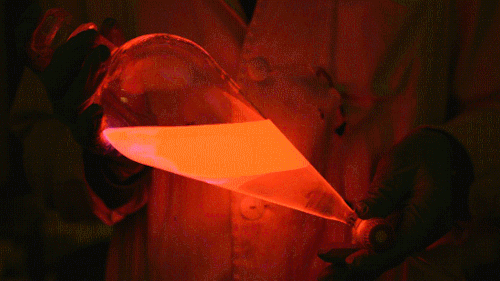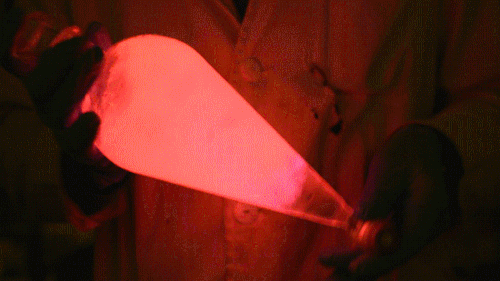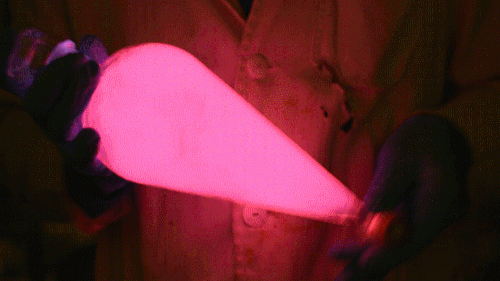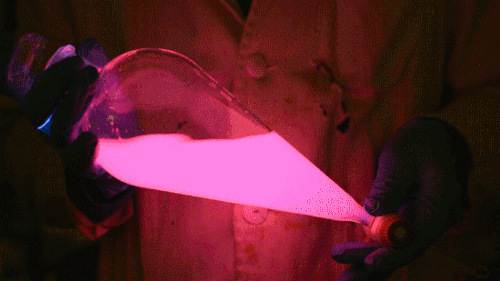

Playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye. The chemistry behind the reaction is something like this:

During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on this video:

COFFEE STAIN UNDER A MICROSCOPE
Vin Kitayama, an artist and environmental researcher, created this image from something fairly mundane: an evaporating drop of espresso. Kitayama placed the drop on a microscope slide and then snapped pictures through a polarized light microscope at 4× magnification. As the coffee dried, solid compounds that were dissolved in the coffee, such as caffeine and chlorogenic acid, started forming small crystals. In the polarized light, these crystals shimmered different colors. The image won 9th place in the Nikon Small World photomicrography competition.
Credit: Vin Kitayama
More Chemistry in Pictures and C&EN content:
U.S. Senators Push for Ban on Caffeine Powder
Caffeinated Cocrystals
Tweaking Coffee’s Flavor Chemistry

Corrugated Liomera - Liomera rugata
This ultra-pinkish crab (actually magenta) is scientifically named Liomera rugata (Decapoda - Xanthidae), a species which inhabits in coral reefs of the Red Sea, Tahiti, Hawaii, Philippines, China Sea, Japan, India and French Polynesia. It is also commonly known as Corrugated Crab due to the visible granules that cover the surface of carapace.
References: [1] - [2]
Photo credit: ©Todd Aki | Locality: Hilo, Hawaii (2014)




Next week I’ll give a presentation on the Researchers Night at Eötvös Loránd University, Hungary with the title: “Chemistry of light and the light of chemistry”.
During this presentation one of my favorite dyes will be also presented: Nile Red. However, just as usual, the 1000 USD/gram price was a bit over our budget, so I had to make it.
The raw product was contaminated with a few impurities, but a fast purification, by simple filtering the mixture through a short column helped a lot and ended up with a +95% pure product.
At first I concentrated the product from a dilute solution on the column as seen on the first pics. It’s interesting to see, that it has a different fluorescence in solution (faint orange fluorescent) and while it’s absorbed on the solid phase (pink, highly fluorescent).
After all the product was on the solid phase, I added another solvent and washed down the pure, HIGHLY FLUORESCENT product. Everything else, what was mainly products of side reactions, stuck at the top of the column as seen on the second pics and the gifs.
Also here is a video from the whole process in HD: https://youtu.be/W0Lk5jkd_B0


The Oldest Ancestor of Modern Birds Has Been Found in China
Ever since the birdlike dinosaur Archaeopteryx was first discovered in 1861, paleontologists have tried to decipher the evolutionary origins of modern birds—the only surviving descendants of the dinosaurs.
Now, paleontologists based out of the Chinese Academy of Sciences (CAS) have reached a new milestone in this quest. The CAS team has discovered the oldest fossils from the Ornithuromorpha group of dinosaurs, the common ancestor of all modern bird species.
The two specimens date back 130 million years to the Early Cretaceous period, when pterosaurs still dominated the skies. They belong to a new species named Archaeornithura meemannae, a feathered wading bird that lived in what is now northeastern China. The CAS team, led by paleontologist Min Wang, published a detailed analysis of the new specimens today in Nature Communications.
Continue Reading.

This week is Antibiotic Awareness Week – learn more about the different types of antibiotics with this graphic!
-
 oneapplepiefromscratchplease reblogged this · 9 years ago
oneapplepiefromscratchplease reblogged this · 9 years ago -
 oneapplepiefromscratchplease liked this · 9 years ago
oneapplepiefromscratchplease liked this · 9 years ago -
 secretporcupine reblogged this · 9 years ago
secretporcupine reblogged this · 9 years ago -
 jesschezz reblogged this · 9 years ago
jesschezz reblogged this · 9 years ago -
 secretporcupine liked this · 9 years ago
secretporcupine liked this · 9 years ago -
 lirio-dendron reblogged this · 9 years ago
lirio-dendron reblogged this · 9 years ago -
 drains-of-heaven liked this · 9 years ago
drains-of-heaven liked this · 9 years ago -
 lvnarsapphic reblogged this · 9 years ago
lvnarsapphic reblogged this · 9 years ago -
 theimmaculategrey-canvas reblogged this · 9 years ago
theimmaculategrey-canvas reblogged this · 9 years ago -
 gottropicana reblogged this · 9 years ago
gottropicana reblogged this · 9 years ago -
 planetofinsects reblogged this · 9 years ago
planetofinsects reblogged this · 9 years ago -
 planetofinsects liked this · 9 years ago
planetofinsects liked this · 9 years ago -
 crapstone liked this · 9 years ago
crapstone liked this · 9 years ago -
 kaitosham reblogged this · 9 years ago
kaitosham reblogged this · 9 years ago -
 anispi liked this · 9 years ago
anispi liked this · 9 years ago -
 the-sleepy-chemist reblogged this · 9 years ago
the-sleepy-chemist reblogged this · 9 years ago -
 allaroundnerd reblogged this · 9 years ago
allaroundnerd reblogged this · 9 years ago -
 fleurdenel-blog liked this · 9 years ago
fleurdenel-blog liked this · 9 years ago -
 thepurestelement reblogged this · 9 years ago
thepurestelement reblogged this · 9 years ago -
 mysoulforhisglory reblogged this · 9 years ago
mysoulforhisglory reblogged this · 9 years ago -
 tinaturninyouout reblogged this · 9 years ago
tinaturninyouout reblogged this · 9 years ago -
 esaspades reblogged this · 9 years ago
esaspades reblogged this · 9 years ago -
 bruisedkid reblogged this · 9 years ago
bruisedkid reblogged this · 9 years ago -
 itaintrocketsciencebub liked this · 9 years ago
itaintrocketsciencebub liked this · 9 years ago -
 sabrina---bee reblogged this · 9 years ago
sabrina---bee reblogged this · 9 years ago -
 sciencetylia reblogged this · 9 years ago
sciencetylia reblogged this · 9 years ago -
 p-p-p-parties reblogged this · 9 years ago
p-p-p-parties reblogged this · 9 years ago -
 p-p-p-parties liked this · 9 years ago
p-p-p-parties liked this · 9 years ago -
 jadeboehmer reblogged this · 9 years ago
jadeboehmer reblogged this · 9 years ago -
 tryingtoremembertobeawesome liked this · 9 years ago
tryingtoremembertobeawesome liked this · 9 years ago -
 kinda-geeky liked this · 9 years ago
kinda-geeky liked this · 9 years ago -
 ctyjohnshopkins reblogged this · 9 years ago
ctyjohnshopkins reblogged this · 9 years ago -
 gentleheart78 liked this · 9 years ago
gentleheart78 liked this · 9 years ago -
 sciencemouse liked this · 9 years ago
sciencemouse liked this · 9 years ago -
 vvjayvv reblogged this · 9 years ago
vvjayvv reblogged this · 9 years ago -
 vvjayvv liked this · 9 years ago
vvjayvv liked this · 9 years ago
60 posts