The-sleepy-chemist - Where Science-y Things Are Posted

More Posts from The-sleepy-chemist and Others
I Recently read About these amazing glow in the dark creatures in the newspapers and thought it was worth sharing 1. Saprobe Panellus Stipticus, Fungi:


Found in Asia, Australia, Europe and North America, the bio-luminescence emitted by the Saprobe fungi that grows on decaying wood...





Beautiful Winged Insects Made of Discarded Circuit Boards by Julie Alice Chappell

KISS OF DEATH
One of the reasons cancer is so hard to defeat is that the body’s immune system has trouble recognizing cancer cells growing among healthy cells. Some scientists want to help. Researchers designed mouse T cells to specifically bind to a protein complex on fibrosarcoma MC57 tumor cell membranes. In this sped-up video, once the T cells (each about 10 μm across) meet their targets, they create holes in the cancer cell membranes using a protein called perforin. Next, the immune cells flood the pierced cells with a rush of cell-killing granules called granzymes. Propidium iodide, a dye the scientists added to the plate of cells, also squeezes in through the hole and starts glowing red when it comes in contact with RNA and DNA inside the cancer cells. This tells the researchers that the cells have been pierced and will soon die. This process takes about 75 minutes in real time.
Credit: Misty Jenkins (Read the paper.)
Related C&EN content:
The Immune System Fights Back
Cancer-Killing Machine

COFFEE STAIN UNDER A MICROSCOPE
Vin Kitayama, an artist and environmental researcher, created this image from something fairly mundane: an evaporating drop of espresso. Kitayama placed the drop on a microscope slide and then snapped pictures through a polarized light microscope at 4× magnification. As the coffee dried, solid compounds that were dissolved in the coffee, such as caffeine and chlorogenic acid, started forming small crystals. In the polarized light, these crystals shimmered different colors. The image won 9th place in the Nikon Small World photomicrography competition.
Credit: Vin Kitayama
More Chemistry in Pictures and C&EN content:
U.S. Senators Push for Ban on Caffeine Powder
Caffeinated Cocrystals
Tweaking Coffee’s Flavor Chemistry


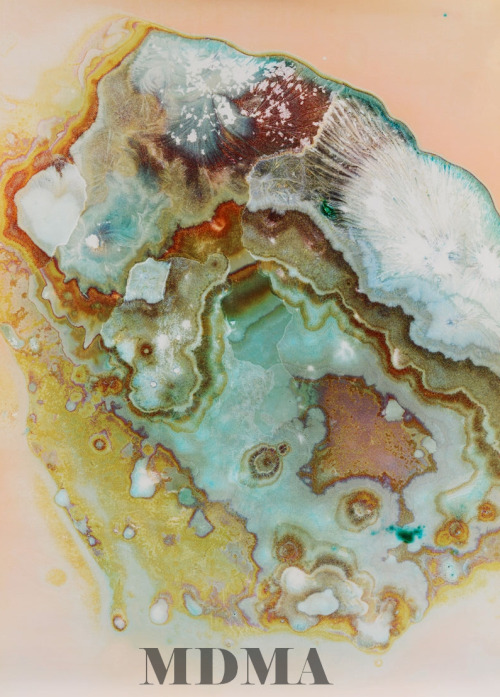







Drugs Under The Microscope
Roses are red Your titriant is pink You failed Go throw your diploma in the sink
Roses are redYour titrant is pinkYou’re not within 2% of the actual valueYou automatically drop a full letter grade on this assignment because achem is a bitch

This Week in Chemistry: Treating cataracts, LED preservatives, nitrogen glaciers, and more! http://goo.gl/56bcZu


The Tsars’s vodka in action. Aqua regia or Царская водка in Russian is a 3/1 mixture of hydrochloric acid and nitric acid.
Upon mixing concentrated hydrochloric acid and concentrated nitric acid a chemical reactions occurs. The product of the reaction is nitrosyl chloride and chlorine as evidenced by the fuming nature and characteristic yellow color of aqua regia. In this case the dissolved copper and other transition metals turned the color of the solution deep green, but the gas over the solution is yellow from the chlorine and nitrous fumes.
Interesting fact about the Nobel prize and the dissolution of gold:
When Nazi Germany occupied Denmark from April 1940, during World War II, György de Hevesy dissolved the gold Nobel Prizes of Max von Laue and James Franck with aqua regia; it was illegal at the time to send gold out of the country, and were it discovered that Laue and Franck had done so to prevent them from being stolen, they could have faced prosecution in Germany. He placed the resulting solution on a shelf in his laboratory at the Niels Bohr Institute. After the war, he returned to find the solution undisturbed and precipitated the gold out of the acid. The Nobel Society then recast the Nobel Prizes using the original gold.
George de Hevesy got his Noble Prize in Chemistry for ”for his work on the use of isotopes as tracers in the study of chemical processes” in 1943.
Max von Laue got his Nobel Prize in Physics for ”for his discovery of the diffraction of X-rays by crystals” in 1914.
James Franck got his Nobel Prize in Physics ”for his discovery of the laws governing the impact of an electron upon an atom” in 1925.
nem sirok csak 65ezren belementek a szemembe
A crowd of 65,000 sings ‘Bohemian Rhapsody’ perfectly while waiting for a Green Day concert



High Current/Amps through metal
Any metal that can conduct low voltage / high amperage electricity acts as a resistor between two electrode wires (as in the case above), which are made out of copper, which has a better conductivity than iron/steel which heats up due to the extreme electrical resistance.
Copper (Cu):
Resistivity: ρ(Ω·m) at 20ºC = 1.68×10−8
Conductivity: σ (S/m) at 20ºC = 5.96×107
Temp. Coefficient: 0.003862 (K−1)
Iron (Fe): (although what you see in the gif is steel, iron comes pretty close)
Resistivity: ρ(Ω m) at 20ºC = 1.00×10−7
Conductivity: σ (S/m) at 20ºC = 1.00×107
Temp. Coefficient: 0.005 (K−1)
Giffed by: rudescience From: This video
-
 jennyjustbeatit liked this · 1 year ago
jennyjustbeatit liked this · 1 year ago -
 careerwithbooks liked this · 1 year ago
careerwithbooks liked this · 1 year ago -
 flucacska reblogged this · 3 years ago
flucacska reblogged this · 3 years ago -
 mind-over-looks liked this · 3 years ago
mind-over-looks liked this · 3 years ago -
 ama3072-blog liked this · 3 years ago
ama3072-blog liked this · 3 years ago -
 thisnightsrevels liked this · 4 years ago
thisnightsrevels liked this · 4 years ago -
 tear-soaked-cheeksdonteverlast reblogged this · 4 years ago
tear-soaked-cheeksdonteverlast reblogged this · 4 years ago -
 silvervinespiderwebs liked this · 4 years ago
silvervinespiderwebs liked this · 4 years ago -
 steblynkaagain liked this · 4 years ago
steblynkaagain liked this · 4 years ago -
 ayumunoya reblogged this · 4 years ago
ayumunoya reblogged this · 4 years ago -
 whinervisitor liked this · 4 years ago
whinervisitor liked this · 4 years ago -
 godofwarotabekaltin liked this · 4 years ago
godofwarotabekaltin liked this · 4 years ago -
 ayumunoya liked this · 4 years ago
ayumunoya liked this · 4 years ago -
 fragdmi liked this · 4 years ago
fragdmi liked this · 4 years ago -
 i-can-kazoo liked this · 4 years ago
i-can-kazoo liked this · 4 years ago -
 otterstudy reblogged this · 5 years ago
otterstudy reblogged this · 5 years ago -
 noafxcklev liked this · 5 years ago
noafxcklev liked this · 5 years ago -
 oceancosmic liked this · 5 years ago
oceancosmic liked this · 5 years ago -
 trailerparkdreams liked this · 5 years ago
trailerparkdreams liked this · 5 years ago -
 sfa-3 reblogged this · 5 years ago
sfa-3 reblogged this · 5 years ago -
 rosebud1218 liked this · 5 years ago
rosebud1218 liked this · 5 years ago -
 onesmoothassendoplasmicreticulum reblogged this · 5 years ago
onesmoothassendoplasmicreticulum reblogged this · 5 years ago -
 onesmoothassendoplasmicreticulum liked this · 5 years ago
onesmoothassendoplasmicreticulum liked this · 5 years ago -
 muslimintp-1999-girl reblogged this · 5 years ago
muslimintp-1999-girl reblogged this · 5 years ago -
 muslimintp-1999-girl liked this · 5 years ago
muslimintp-1999-girl liked this · 5 years ago -
 creeomyself reblogged this · 5 years ago
creeomyself reblogged this · 5 years ago -
 creeomyself liked this · 5 years ago
creeomyself liked this · 5 years ago -
 mekapeka liked this · 5 years ago
mekapeka liked this · 5 years ago -
 alyssa-anne reblogged this · 6 years ago
alyssa-anne reblogged this · 6 years ago -
 alyssa-anne liked this · 6 years ago
alyssa-anne liked this · 6 years ago -
 bioqueen reblogged this · 6 years ago
bioqueen reblogged this · 6 years ago -
 yakutyanochka liked this · 6 years ago
yakutyanochka liked this · 6 years ago -
 cervicalwelding liked this · 6 years ago
cervicalwelding liked this · 6 years ago -
 bittyasiankit reblogged this · 6 years ago
bittyasiankit reblogged this · 6 years ago -
 bittyasiankit liked this · 6 years ago
bittyasiankit liked this · 6 years ago -
 mandalayogi7 liked this · 6 years ago
mandalayogi7 liked this · 6 years ago -
 newtyp liked this · 6 years ago
newtyp liked this · 6 years ago -
 di-vagueando reblogged this · 6 years ago
di-vagueando reblogged this · 6 years ago -
 thecookieraakshas liked this · 6 years ago
thecookieraakshas liked this · 6 years ago -
 dearkurisu liked this · 6 years ago
dearkurisu liked this · 6 years ago
60 posts