Astronomers Discover An Entirely New Kind Of Galaxy

Astronomers Discover an Entirely New Kind of Galaxy
Astronomers at the University of Minnesota Duluth and the North Carolina Museum of Natural Sciences have identified a new class of ring galaxy. Named PGC 1000714, it features an elliptical core with not one, but two outer rings. It’s the only known galaxy of its kind in the known universe.
More Posts from Contradictiontonature and Others

After the news of an accident in a French drug trial on Friday, you might be wondering what drug trials entail. Here’s a summary sheet on the drug discovery process to clear things up! http://wp.me/p4aPLT-1EZ

4 new elements added to the periodic table
The seventh row of the Periodic Table of Elements is now complete, rendering all textbooks out of date. The discovered elements don’t have permanent names yet, but their atomic numbers are 113, 115, 117 and 118.
Livermore Lab scientists and international collaborators have officially discovered three of the four new elements: 115, 117 and 118. The illustration above is of 117, tentatively named ununseptium or Uus.
The new elements’ existence was confirmed by further experiments that reproduced them — however briefly. Element 113, for instance, exists for less than a thousandth of a second.
Learn more about the new elements

Vexing Virus Shields
Around 36 million people worldwide have HIV. Scientists are trying to develop an HIV vaccine using antibodies, molecules our bodies make to target and tag invading particles. Researchers have found that rabbits exposed to a strain of HIV produce antibodies targeting a specific part of the virus: a hole in its glycan shield. This shield consists of sugar molecules attached to the outside of the virus, protecting it from attack – the hole is thus a gap in its defences. However, although most HIV strains have a hole in their glycan shields, not many strains have one in the same position as was identified, so different antibodies would need to be developed. The image shows how much certain parts of the glycan shield are conserved between different HIV strains – red being 90–100%, and green 50–60%. This is a step towards vaccines, but the shield remains a challenge in vaccine development.
Written by Esther Redhouse White
Image by Sergey Menis, Laura McCoy and James Voss
The Scripps Research Institute, USA and University of Amsterdam, The Netherlands
Image copyright held by original authors
Research published in Cell Reports, August 2016
Published in eLife, June 2016
You can also follow BPoD on Twitter and Facebook
Complement Pathways

Components of complement pathways of the immune system.
Classical Pathway: binds to the pathogen surface
C1 binds to phosphocholine on bacteria, which activates C1r to cleave C1s.
Activated C1s cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by C1s, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
MB-Lectin Pathway: uses mannin-binding lectin to bind to mannose-containing carbohydrates on the pathogen surface
Mannin-binding lectin (MBL) binds to the pathogen surface and activates MASP-2.
MASP-2 cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by MASP-2, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
Alternative Pathway: binds to the pathogen surface with spontaneously activated complement, amplifies C3b
C3b deposited by the C3 convertase binds to factor B.
Factor B is cleaved by factor D into Ba and Bb, forming the C3bBb complex.
The C3bBb complex cleaves C3 into C3a and C3b.
C3 spontaneously hydrolyzes to C3(H2O).
C3(H2O) binds to factor B, and factor D cleaves factor B.
Upon factor B cleavage, the C3(H2O)Bb complex is formed.
The C3(H2O)Bb complex cleaves C3 into C3a and C3b.
Factor B binds to C3b on the surface and is cleaved to Bb.

It was one of the very first motion pictures ever made: a galloping mare filmed in 1878 by the British photographer Eadweard Muybridge, who was trying to learn whether horses in motion ever become truly airborne.
More than a century later, that clip has rejoined the cutting edge. It is now the first movie ever to be encoded in the DNA of a living cell, where it can be retrieved at will and multiplied indefinitely as the host divides and grows.
The advance, reported on Wednesday in the journal Nature by researchers at Harvard Medical School, is the latest and perhaps most astonishing example of the genome’s potential as a vast storage device.
Continue Reading.

A New Way to Cross the Blood–Brain Barrier - A Mental Unblock
The brain presents a unique challenge for medical treatment: it is locked away behind an impenetrable layer of tightly packed cells. Although the blood-brain barrier prevents harmful chemicals and bacteria from reaching our control center, it also blocks roughly 95 percent of medicine delivered orally or intravenously. As a result, doctors who treat patients with neurodegenerative diseases, such as Parkinson’s, often have to inject drugs directly into the brain, an invasive approach that requires drilling into the skull.
Some scientists have had minor successes getting intravenous drugs past the barrier with the help of ultrasound or in the form of nanoparticles, but those methods can target only small areas. Now neuroscientist Viviana Gradinaru and her colleagues at the California Institute of Technology show that a harmless virus can pass through the barricade and deliver treatment throughout the brain.
Gradinaru’s team turned to viruses because the infective agents are small and adept at entering cells and hijacking the DNA within. They also have protein shells that can hold beneficial deliveries, such as drugs or genetic therapies. To find a suitable virus to enter the brain, the researchers engineered a strain of an adeno-associated virus into millions of variants with slightly different shell structures. They then injected these variants into a mouse and, after a week, recovered the strains that made it into the brain. A virus named AAV-PHP.B most reliably crossed the barrier.
Next the team tested to see if AAV-PHP.B could work as a potential vector for gene therapy, a technique that treats diseases by introducing new genes into cells or by replacing or inactivating genes already there. The scientists injected the virus into the bloodstream of a mouse. In this case, the virus was carrying genes that encoded green fluorescent proteins. So if the virus made it to the brain and the new DNA was incorporated in neurons, the success rate could be tracked via a green glow on dissection. Indeed, the researchers observed that the virus infiltrated most brain cells and that the glowing effects lasted as long as one year. The results were recently published in Nature Biotechnology.
In the future, this approach could be used to treat a range of neurological diseases. “The ability to deliver genes to the brain without invasive methods will be extremely useful as a research tool. It has tremendous potential in the clinic as well,” says Anthony Zador, a neuroscientist who studies brain wiring at Cold Spring Harbor Laboratory. Gradinaru also thinks the method is a good candidate for targeting areas other than the brain, such as the peripheral nervous system. The sheer number of peripheral nerves has made pain treatment for neuropathy difficult, and a virus could infiltrate them all.
Image Credit: Thomas Fuchs
Source: Scientific American (By Monique Brouillette)
Toxic Alzheimer’s Protein Spreads Through Brain Via Extracellular Space
A toxic Alzheimer’s protein can spread through the brain—jumping from one neuron to another—via the extracellular space that surrounds the brain’s neurons, suggests new research from Karen Duff, PhD, and colleagues at Columbia University Medical Center.

(Image caption: Orange indicates where tau protein has traveled from one neuron to another. Credit: Laboratory of Karen Duff, PhD)
The spread of the protein, called tau, may explain why only one area of the brain is affected in the early stages of Alzheimer’s but multiple areas are affected in later stages of the disease.
“By learning how tau spreads, we may be able to stop it from jumping from neuron to neuron,” says Dr. Duff. “This would prevent the disease from spreading to other regions of the brain, which is associated with more severe dementia.”
The idea the Alzheimer’s can spread through the brain first gained support a few years ago when Duff and other Columbia researchers discovered that tau spread from neuron to neuron through the brains of mice.
In the new study, lead scientist Jessica Wu, PhD, of the Taub Institute discovered how tau travels by tracking the movement of tau from one neuron to another. Tau, she found, can be released by neurons into extracellular space, where it can be picked up by other neurons. Because tau can travel long distances within the neuron before its release, it can seed other regions of the brain.
“This finding has important clinical implications,” explains Dr. Duff. “When tau is released into the extracellular space, it would be much easier to target the protein with therapeutic agents, such as antibodies, than if it had remained in the neuron.”
A second interesting feature of the study is the observation that the spread of tau accelerates when the neurons are more active. Two team members, Abid Hussaini, PhD, and Gustavo Rodriguez, PhD, showed that stimulating the activity of neurons accelerated the spread of tau through the brain of mice and led to more neurodegeneration.
Although more work is needed to examine whether those findings are relevant for people, “they suggest that clinical trials testing treatments that increase brain activity, such as deep brain stimulation, should be monitored carefully in people with neurodegenerative diseases,” Dr. Duff says.
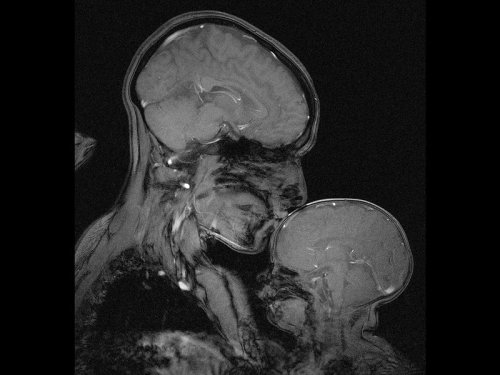
Neuroscientist captures an MRI of a mother and child
Professor Rebecca Saxe (MIT) has taken the first ever MR image of a mother and child.
“This picture is an MR image of a mother and a child that I made in my lab at MIT. You might see it as sweet and touching… an image of universal love. We can’t see clothes or hairstyles or even skin colour. From what we do see, the biology and the brains, this could be any mother and child or even father and child at any time and place in history; having an experience that any human can recognise.
Or you might see it as disturbing, a reminder that our human bodies are much too fragile as houses for ourselves. MRI’s are usually medical images and often bad news. Each white spot in that picture is a blood vessel that could clog, each tiny fold of those brains could harbour a tumour. The baby’s brain maybe looks particularly vulnerable pressed against the soft thin shell of its skull.
I see those things, universal emotions and frightening fragility but I also see one of the most amazing transformations in biology.”
Quotes have been taken from a TEDx talk given by Professor Saxe explaining the story behind the above picture.
-
 thenaturaldisesther liked this · 7 years ago
thenaturaldisesther liked this · 7 years ago -
 stoopsitter reblogged this · 7 years ago
stoopsitter reblogged this · 7 years ago -
 vivalasestrellas reblogged this · 7 years ago
vivalasestrellas reblogged this · 7 years ago -
 vivalasestrellas liked this · 7 years ago
vivalasestrellas liked this · 7 years ago -
 jane-austen-powers liked this · 7 years ago
jane-austen-powers liked this · 7 years ago -
 dakatndahat liked this · 7 years ago
dakatndahat liked this · 7 years ago -
 funcoolmathgames reblogged this · 7 years ago
funcoolmathgames reblogged this · 7 years ago -
 thesherlokidwhovian liked this · 8 years ago
thesherlokidwhovian liked this · 8 years ago -
 three-six-bee-no-scope liked this · 8 years ago
three-six-bee-no-scope liked this · 8 years ago -
 podly-sok-pomaranczowy liked this · 8 years ago
podly-sok-pomaranczowy liked this · 8 years ago -
 cinnamonrollsapphic reblogged this · 8 years ago
cinnamonrollsapphic reblogged this · 8 years ago -
 cinnamonrollsapphic liked this · 8 years ago
cinnamonrollsapphic liked this · 8 years ago -
 captainjanegay reblogged this · 8 years ago
captainjanegay reblogged this · 8 years ago -
 luxurycomedyqueer liked this · 8 years ago
luxurycomedyqueer liked this · 8 years ago -
 segwey reblogged this · 8 years ago
segwey reblogged this · 8 years ago -
 afairfight reblogged this · 8 years ago
afairfight reblogged this · 8 years ago -
 mavka-inthewoods liked this · 8 years ago
mavka-inthewoods liked this · 8 years ago -
 omg-bookworm liked this · 8 years ago
omg-bookworm liked this · 8 years ago -
 saintofpants liked this · 8 years ago
saintofpants liked this · 8 years ago -
 do-not-doughnut-with-cats-blog liked this · 8 years ago
do-not-doughnut-with-cats-blog liked this · 8 years ago -
 dontspillmyskittles reblogged this · 8 years ago
dontspillmyskittles reblogged this · 8 years ago -
 ianjingram reblogged this · 8 years ago
ianjingram reblogged this · 8 years ago -
 styxxus liked this · 8 years ago
styxxus liked this · 8 years ago -
 stoleandromance reblogged this · 8 years ago
stoleandromance reblogged this · 8 years ago -
 sweetlyminiaturesublime reblogged this · 8 years ago
sweetlyminiaturesublime reblogged this · 8 years ago -
 sweetlyminiaturesublime liked this · 8 years ago
sweetlyminiaturesublime liked this · 8 years ago -
 sweetsilentsteps reblogged this · 8 years ago
sweetsilentsteps reblogged this · 8 years ago -
 larkofthesky liked this · 8 years ago
larkofthesky liked this · 8 years ago -
 missbutterflywitchlady reblogged this · 8 years ago
missbutterflywitchlady reblogged this · 8 years ago -
 cuter-than-hell liked this · 8 years ago
cuter-than-hell liked this · 8 years ago -
 troglobite liked this · 8 years ago
troglobite liked this · 8 years ago -
 bullydogs liked this · 8 years ago
bullydogs liked this · 8 years ago -
 ussenterprisse liked this · 8 years ago
ussenterprisse liked this · 8 years ago -
 softjjong reblogged this · 8 years ago
softjjong reblogged this · 8 years ago -
 paladinical liked this · 8 years ago
paladinical liked this · 8 years ago -
 littleclouds reblogged this · 8 years ago
littleclouds reblogged this · 8 years ago -
 astro-kersten liked this · 8 years ago
astro-kersten liked this · 8 years ago -
 trinity996 liked this · 8 years ago
trinity996 liked this · 8 years ago -
 catwitching liked this · 8 years ago
catwitching liked this · 8 years ago -
 queermil reblogged this · 8 years ago
queermil reblogged this · 8 years ago -
 thestrongestsheep-blog reblogged this · 8 years ago
thestrongestsheep-blog reblogged this · 8 years ago -
 thestrongestsheep-blog liked this · 8 years ago
thestrongestsheep-blog liked this · 8 years ago -
 connotationofinfinity reblogged this · 8 years ago
connotationofinfinity reblogged this · 8 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts





