4 New Elements Added To The Periodic Table

4 new elements added to the periodic table
The seventh row of the Periodic Table of Elements is now complete, rendering all textbooks out of date. The discovered elements don’t have permanent names yet, but their atomic numbers are 113, 115, 117 and 118.
Livermore Lab scientists and international collaborators have officially discovered three of the four new elements: 115, 117 and 118. The illustration above is of 117, tentatively named ununseptium or Uus.
The new elements’ existence was confirmed by further experiments that reproduced them — however briefly. Element 113, for instance, exists for less than a thousandth of a second.
Learn more about the new elements
More Posts from Contradictiontonature and Others

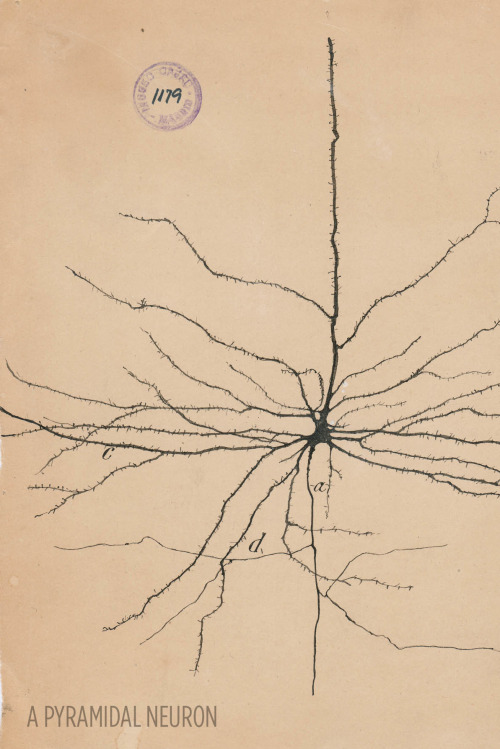



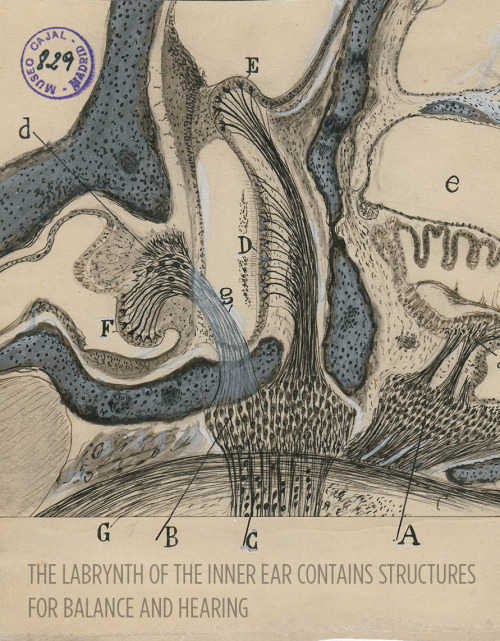


Art and science in beautiful conversation!
Here’s a 30-something Santiago Ramón y Cajal hanging out in his library:

For more, check out this article or visit the Weisman Art Museum in Minnesota before May 21.
Images courtesy of Instituto Cajal del Consjo Superior de Investigaciones Científicas, Madrid
The time will come when diligent research over long periods will bring to light things which now lie hidden. A single lifetime, even though entirely devoted to the sky, would not be enough for the investigation of so vast a subject… And so this knowledge will be unfolded through long successive ages. There will come a time when our descendants will be amazed that we did not know things that are so plain to them… Many discoveries are reserved for ages still to come, when memory of us will have been effaced. Our universe is a sorry little affair unless it has something for every age to investigate… Nature does not reveal her mysteries once and for all.
Seneca
(via scienceisbeauty)

Genes to Brains
Over the years scientists have carefully mapped the brain, figuring out which regions perform different functions. Techniques such as functional MRI can show exactly which parts are active when people are doing all kinds of other tasks. Detailed microscopy and brain-scanning studies have traced the intricate network of connections between nerve cells in the brain, revealing the inner wiring of this powerful biological computer. But until now, nobody has tried to link patterns of gene activity into this functional and structural information. For the first time, researchers have generated this map of the brain, with each colour highlighting a particular group of genes that seem to be linked to that region. There are many variations in human genes that can influence traits and conditions affecting the brain, such as intelligence or autism, and this is the first step towards figuring out exactly how these genetic variations might exert their effects.
Written by Kat Arney
Image from work by Qian Peng and colleagues
Department of Human Biology, J. Craig Venter Institute, and Multimodal Imaging Laboratory, Department of Radiology, University of California San Diego, La Jolla, CA, USA
Image originally published under a Creative Commons Licence (BY 4.0)
Published in PLOS Genetics, July 2016
You can also follow BPoD on Twitter and Facebook


Leopard shark makes world-first switch from sexual to asexual reproduction
A leopard shark in an Australian aquarium has reproduced asexually after being separated from her mate.
It is the first reported case of a shark switching from sexual to asexual or parthenogenetic reproduction and only the third reported case among all vertebrate species.
The leopard shark, Leonie, was captured in the wild in 1999 and introduced to a male shark at the Reef HQ aquarium in Townsville, Queensland, in 2006. Leopard sharks are also known as zebra sharks.
One of the baby sharks born to leopard sharks at Reef HQ aquarium in Townsville, who have produced live young through asexual reproduction. Photograph: Tourism and Events Queensland
Leonie, the world’s first shark known to have switched from sexual to asexual reproduction, at Reef HQ aquarium in Townsville. Photograph: Tourism and Events Queensland

For #WorldBeeDay, here’s a look at the chemistry behind the honey some bees produce: https://ift.tt/2GV5qtq https://ift.tt/2LJpsIe
Neuroscientists’ Study Sheds Light on How Words Are Represented in the Brain
Reading is a relatively modern and uniquely human skill. For this reason, visual word recognition has been a puzzle for neuroscientists because the neural systems responsible for reading could not have evolved for this purpose. “The existence of brain regions dedicated to reading has been fiercely debated for almost 200 years,” said Avniel Ghuman, an assistant professor in the University of Pittsburgh Department of Neurological Surgery. “Wernicke, Dejerine, and Charcot, among the most important and influential neurologists and neuroscientists of the 19th century, debated whether or not there was a visual center for words in the brain.”

In recent years, much of this debate has centered on the left mid-fusiform gyrus, which some call the visual word form area. A recent study by Pitt neuroscience researchers addresses this debate and sheds light on our understanding of the neurobiology of reading.
In a study published July 19 in the Proceedings of the National Academy of Sciences, Ghuman, Elizabeth Hirshorn of Pitt’s Learning Research and Development Center (LRDC), and colleagues from the Department of Psychology and Center for the Neural Basis of Cognition used direct neural recordings and brain stimulation to study the role of the visual word form area in reading in four epileptic patients. The patients chose surgical treatment for their drug-resistant epilepsy and volunteered to participate in the research study. As part of the surgical treatment, neurosurgeons implanted electrodes in the patients’ visual word form area, providing an unprecedented opportunity to understand how the brain recognizes printed words.
First, painless electrical brain stimulation was used through the electrodes to disrupt the normal functioning of the visual word form area, which adversely affected the patients’ ability to read words. One patient dramatically misperceived letters, and another felt that there were words and parts of words present that were not in what she was reading. Stimulation to this region did not disrupt their ability to name objects or faces. A brief video of the stimulation can be seen here.
In addition to stimulating through these electrodes, the activity from the area was recorded while the patients read words. Using techniques from machine learning to analyze the brain activity that evolved over a few hundred milliseconds from this region, the researchers could tell what word a patient was reading at a particular moment. This suggests that neural activity in the area codes knowledge about learned visual words that can be used to discriminate even words that are only one letter different from one another (for example, “hint” and “lint”).
“This study shows that the visual word form area is exquisitely tuned to the fine details of written words and that this area plays a critical role in refining the brain’s representation of what we are reading. The disrupted word and letter perception seen with stimulation provides direct evidence that the visual word form area plays a dedicated role in skilled reading,” said Hirshorn. “These results also have important implications for understanding and treating reading disorders. The activity in the visual word form area, along with its interactions with other brain areas involved in language processing, could be a marker for proficient reading. Having a better understanding of this neural system could be critical for diagnosing reading disorders and developing targeted therapies.”
“It is exciting that with modern brain-recording techniques and advanced analysis methods, we are finally able to start answering questions about the brain and the mind that people have asked for centuries and contribute to our understanding of reading disorders,” said Ghuman.










Giant Artwork Reflects The Gorgeous Complexity of The Human Brain
The new work at The Franklin Institute may be the most complex and detailed artistic depiction of the brain ever.
Your brain has approximately 86 billion neurons joined together through some 100 trillion connections, giving rise to a complex biological machine capable of pulling off amazing feats. Yet it’s difficult to truly grasp the sophistication of this interconnected web of cells.
Now, a new work of art based on actual scientific data provides a glimpse into this complexity.
The 8-by-12-foot gold panel, depicting a sagittal slice of the human brain, blends hand drawing and multiple human brain datasets from several universities. The work was created by Greg Dunn, a neuroscientist-turned-artist, and Brian Edwards, a physicist at the University of Pennsylvania, and goes on display at The Franklin Institute in Philadelphia.
“The human brain is insanely complicated,” Dunn said. “Rather than being told that your brain has 80 billion neurons, you can see with your own eyes what the activity of 500,000 of them looks like, and that has a much greater capacity to make an emotional impact than does a factoid in a book someplace.”
To reflect the neural activity within the brain, Dunn and Edwards have developed a technique called micro-etching: They paint the neurons by making microscopic ridges on a reflective sheet in such a way that they catch and reflect light from certain angles. When the light source moves in relation to the gold panel, the image appears to be animated, as if waves of activity are sweeping through it.
First, the visual cortex at the back of the brain lights up, then light propagates to the rest of the brain, gleaming and dimming in various regions — just as neurons would signal inside a real brain when you look at a piece of art.
That’s the idea behind the name of Dunn and Edwards’ piece: “Self Reflected.” It’s basically an animated painting of your brain perceiving itself in an animated painting.
To make the artwork resemble a real brain as closely as possible, the artists used actual MRI scans and human brain maps, but the datasets were not detailed enough. “There were a lot of holes to fill in,” Dunn said. Several students working with the duo explored scientific literature to figure out what types of neurons are in a given brain region, what they look like and what they are connected to. Then the artists drew each neuron.
Dunn and Edwards then used data from DTI scans — a special type of imaging that maps bundles of white matter connecting different regions of the brain. This completed the picture, and the results were scanned into a computer. Using photolithography, the artists etched the image onto a panel covered with gold leaf.
“A lot of times in science and engineering, we take a complex object and distill it down to its bare essential components, and study that component really well” Edwards said. But when it comes to the brain, understanding one neuron is very different from understanding how billions of neurons work together and give rise to consciousness.
“Of course, we can’t explain consciousness through an art piece, but we can give a sense of the fact that it is more complicated than just a few neurons,” he added.
The artists hope their work will inspire people, even professional neuroscientists, “to take a moment and remember that our brains are absolutely insanely beautiful and they are buzzing with activity every instant of our lives,” Dunn said. “Everybody takes it for granted, but we have, at the very core of our being, the most complex machine in the entire universe.”
Image 1: A computer image of “Self Reflected,” an etching of a human brain created by artists Greg Dunn and Brian Edwards.
Image 2: A close-up of the cerebellum in the finished work.
Image 3: A close-up of the motor cortex in the finished work.
Image 4: This is what “Self Reflected” looks like when it’s illuminated with all white light.
Image 5: Pons and brainstem close up.
Image 6: Putkinje neurons - color encodes reflective position in microetching.
Image 7: Primary visual cortex in the calcarine fissure.
Image 8: Basal ganglia and connected circuitry.
Image 9: Parietal cortex.
Image 10: Cerebellum.
Credit for all Images: Greg Dunn - “Self Reflected”
Source: The Huffington Post (by Bahar Gholipour)

Anti-microbial Peptides: Proteins that Pack a Punch
Antimicrobial resistance is a growing concern and it is currently estimated that approximately 2 million people are infected annually with serious infections that show antibiotic resistance to some degree. This contributes to the mortality of 23, 000 people with many more suffering severe complications as a direct result of antibiotic resistant infections. The economic burden on the US is thought to exceed $20 billion simply on health care bills alone, and a further $35 billion due to a societal loss in work based productivity (1).
The spread of antibiotic resistance is now widely believed to be a direct result of the anthropogenic release of antibiotics into the biosphere. We are now faced with the dilemma of how to treat these infections. In previous articles, I’ve talked largely about bacteriophages and how they are one possible solution to this complex problem. This article will introduce you to another class of antimicrobial agents, aptly called antimicrobial peptides (AMPs).
What are Antimicrobial Peptides?
Proteins are found ubiquitously throughout all cellular life and are like the mechanical parts of a car, helping your cells carry out a vast array of functions every single day. Peptides are small proteins that contain two or more amino acids joined by peptide bonds. Anyone who is familiar with biochemistry will be aware of the sheer diversity found amongst these versatile molecules. Needless to say, it should not be surprising that there are a large class of proteins involved in offensive cellular warfare. They are found widely in all domains of life and have evolved to give a cell a competitive advantage over its nastier neighbours.
Without getting too bogged down with the biochemistry, AMPs are characterised by their overall properties. AMPs that share common structural features will also have a similar function when targeting a cell. The diversity amongst these proteins can be seen in Figure 1, which shows some examples from the four classes of AMPs. The class I AMPs, the lantibiotics for example, all contain similar motifs which assign them a similar job. AMPs can range from anywhere between 6 to >59 amino acids, but are generally considered to be small proteins (2). They generally have a rather amphipathic nature and feature both positive and negative charges.
These peptides may have a number of rare (Figure 1), modified amino acids. The lanthionines are a class of AMP that contain lanthionine rings made from dehydrated serine and threonine residues connected by thioether cross-links. This happens after the protein leaves the ribosome and gives the protein some very unique properties which will be explained later in the article (3).
Figure 1. The four classes of AMPs, showing common examples in each class. Rare, modified amino acids are indicated by coloured circles with the three letter codes indicating the name of the residue. Thioether cross-links are indicated by an S coordinated by two black lines (3).
Implications for the Pharmaceutical industry
Our antibiotic pipeline is drying up (Figure 2), with few new drugs being approved by the Food and Drug Administration. Identifying novel antibiotics is a tedious process that requires a lot of time and effort from drug companies, which they are not willing to do. The reason for this boils down to economic reasons, as antibiotics are just not worth the investment. Unlike other drugs such as statins, antibiotics are only used for short periods of time by a patient. One course of treatment therefore doesn’t return a massive profit for the company. The second issue antibiotics face is that resistance to them occurs rapidly after they are put into circulation, so the company is not likely to get much use out of the drug. Therefore we need to find a new source for our antimicrobials. This is where the AMPs come in.
Currently, nearly 900 AMPs have been identified and characterised with many more undiscovered (2). They are an untapped source of drug discovery and they exhibit numerous benefits over their antibiotic cousins. As they are proteins, they have a genetic origin, which could provide an amenable platform for further development through random mutagenesis. This could produce a vast library of antimicrobial compounds (4,5), drastically improving our options for therapy.
Figure 2. Graph showing the steady decline in antibiotic development from 1980 to 2012 (1)
Nisin; not so nice if you’re a bacterial cell
AMPs were discovered in the 1930s although their use in the health industry has been fairly limited, resulting from the sheer difficulty and cost of manufacturing and purifying proteins on a large scale. The bacterially produced lantibiotics are by far the most well studied AMPs and have the most potential for the pharmaceutical industry. Nisin (E234) is the most well studied lantibiotic (Refer to Figure 1, Class I) and is produced by the bacterium Lactococcus lactis (6).
It shows broad spectrum activity on a large number of Gram-positive bacteria including other lactic acid bacteria, which has made it a coveted preservative in food processing. Currently it is added to cheeses, meats and beverages to extend shelf life and prevent the growth of spoilage organisms including spore forming bacteria such as Clostridium botulinum (6). The lantibiotics have also proven their capabilities for treating the clinically relevant pathogens methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci (7). They are also seen to have similar levels of activity as antibiotics and express low levels of toxicity to mammalian cells. Nisin exhibits poor oral availability making it more appealing as a topical agent or for intravenous application but there are also intentions to use it as a sterilising agent for catheters and medical equipment to help reduce the risk of infection (3).
So could lantibiotics like nisin be a good solution to antimicrobial resistance? Well more compelling evidence for nisin is that resistance has not been thoroughly documented. Nisin has been applied in sub-therapeutic concentrations in the food industry since the 1960s but still mostly retains its bactericidal ability. Resistance has been achieved artificially in the lab (discussed later in the article), but due to the mechanism of some lantibiotics including nisin, resistance is thought to be unlikely (6).
The mechanism behind nisin’s potency
Unlike animal cells, generally bacterial membranes have an overall negative charge and lack cholesterol (8). Nisin contains a high proportion of the positively charged (basic) amino acids lysine and arginine. These positive charges allow the protein to interact with the negative charges commonly associated with bacterial cell membranes (2). Nisin is good at aligning against Gram-positive bacterial membranes, where they multimerise to form short-lived pores (Figure 3). Hydrophobic regions help the protein to insert into the membrane and stabilise the pore (2), which allows the transport of ATP, ions and amino acids, eliminating the cellular membrane potential (9).
Nisin has a second trick up its sleeve. Its C-terminal, the portion of the protein containing the lanthionine ring motifs, allows it to latch onto the important membrane component lipid II (Figure 3). Lipid II is a precursor for peptidoglycan; the cell wall strengthening polymer found in both Gram-positive and Gram-negative bacteria. It is a common target for antibiotics including penicillin and vancomycin, which both target different stages of its synthesis. It helps to maintain the cell structure and prevents it from bursting under high osmotic pressure. When nisin binds to lipid II, it sequesters this molecule from the enzymes that catalyse its addition to growing peptidoglycan chains. Binding lipid II also helps to stabilise the transmembrane pores, further damaging the cell. As a result, not only is the cell wall weakened, but the cell loses its metabolic capabilities, through the loss of charged molecules.
The dual targeting system of nisin is thought to be the reason why resistance to nisin has not be well documented (10). The two processes are completely physiologically separate, and therefore to develop resistance, the bacteria would have to develop two unrelated mutations to counteract the effects of nisin.
Figure 3. Diagram showing the mechanism of several lantibiotics including nisin. AMPs are represented by lines made with clear circles. Phospholipids represented by green circles with tails. Lipid II is represented by orange hexagons (3).
What do we know about resistance towards nisin?
There are several proposed means by which an organism can be resistance to a toxin. Firstly, an organism may have innate immunity to a toxin simply because of its physiology. We see this largely in the Gram-negative bacteria towards nisin. The lipopolysaccharide (LPS) layer found on the outside of their cell wall provides protection against nisin and it has been shown that the oligosaccharides found within the core region of this structure greatly improve protection against nisin. It is believed that this is because metal ions are sequestered within this layer, adding additional positive charges to the site. Such charges would help to prevent nisin from aligning with the cell membrane (11). Removing these metal ions by sequestering them sensitises Gram-negative bacteria to nisin.
Emergent resistance is the type of resistance that should concern us the most, as it is the reason why we are now faced with the problem of antimicrobial resistance. It involves the acquisition of mutations or DNA that help confer tolerance to stress resulting from the action of a toxin (12). Although currently only produced in the laboratory, experiments carried out on the tolerance of clinically relevant bacteria towards nisin are crucial in highlighting the future of implementing an antimicrobial.
Resistance mechanisms have been documented in several bacteria including the causative agent of listeriosis, Listeria monocytogenes. Although not fully understood, changes in membrane composition have been attributed for the decreased susceptibility in resistant strains. In resistant strains, the bacterial membrane is composed of less negatively charged phospholipids. Similarly to sequestering metal ions near the membrane, this alters the overall net charge, helping to repel nisin.
The number of long chain fatty acids within its membrane is increased helping to reducing fluidity. This is believed to play a role in preventing nisin from inserting itself into the membrane. Studies show that nisin resistant strains were also less susceptible to cell wall acting components such as lysozyme and cell wall acting antibiotics. They did not identify the phenotypic change that gave additional protection, but this does indicate that a number of defence mechanisms are involved in defending cells against environmental stress from nisin (13).
Conclusion:
So could AMPs like nisin possibly serve as a replacement to our current armamentarium of antibiotics? AMPs are a largely untapped source of antimicrobials with many more still to be identified. AMPs may therefore serve as a new source of antimicrobials to help relieve the stress exerted on microorganisms by antibiotics. We have seen that nisin is an effective antimicrobial against a wide range of Gram-positive bacteria including spore forming bacteria. The dual-action of nisin challenges bacterial cells making it difficult for them to develop resistance. However, lab-based experiments have shown that it is possible to generate resistant strains showing the tenacity of bacteria to adapt to such potent environmental stresses. To learn from our previous mistakes with antibiotics, more responsible practices would need to be applied. Using combination therapy or rotating drug usage, as done with pesticides, could help further prevent resistance. Where they are likely to be applied in high concentration (in medical settings and agriculture), combination therapies should be used to further reduce the likelihood of resistance.
1. CDC. Antibiotic resistance threats. US Dep Healh Hum Serv. 2013;22–50.
2. Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol [Internet]. 2005;3(3):238–50. Available from: http://www.nature.com/doifinder/10.1038/nrmicro1098
3. Dischinger J, Basi Chipalu S, Bierbaum G. Lantibiotics: Promising candidates for future applications in health care. Int J Med Microbiol [Internet]. Elsevier GmbH.; 2014;304(1):51–62. Available from: http://dx.doi.org/10.1016/j.ijmm.2013.09.003
4. Field D, Begley M, O’Connor PM, Daly KM, Hugenholtz F, Cotter PD, et al. Bioengineered Nisin A Derivatives with Enhanced Activity against Both Gram Positive and Gram Negative Pathogens. PLoS One. 2012;7(10).
5. Hilpert K, Volkmer-Engert R, Walter T, Hancock REW. High-throughput generation of small antibacterial peptides with improved activity. Nat Biotechnol. 2005;23(8):1008–12.
6. van Heel AJ, Montalban-Lopez M, Kuipers OP. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin Drug Metab Toxicol. 2011;7(6):675–80.
7. Barbosa J, Caetano T, Mendo S. Class I and Class II Lanthipeptides Produced by Bacillus spp. J Nat Prod [Internet]. 2015;151008121848005. Available from: http://pubsdc3.acs.org/doi/10.1021/np500424y
8. Neumann A, Berends ETM, Nerlich A, Molhoek EM, Gallo RL, Meerloo T, et al. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem J [Internet]. 2014 Nov 15 [cited 2014 Oct 28];464(1):3–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25181554
9. Kordel M, Schuller F, Sahl HG. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 1989;244(1):99–102.
10. Islam MR, Nagao J, Zendo T, Sonomoto K. Antimicrobial mechanism of lantibiotics. Biochem Soc Trans [Internet]. 2012;40(6):1528–33. Available from: http://www.biochemsoctrans.org/bst/040/bst0401528.htm
11. Stevens K a., Sheldon BW, Klapes N a., Klaenhammer TR. Nisin treatment for inactivation of Salmonella species and other gram- negative bacteria. Appl Environ Microbiol. 1991;57(12):3613–5.
12. Kaur G, Malik RK, Mishra SK, Singh TP, Bhardwaj A, Singroha G, et al. Nisin and class IIa bacteriocin resistance among Listeria and other Foodborne pathogens and spoilage bacteria. Microb Drug Resist. 2011;17(2).
13. Crandall AD, Montville TJ. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998

At last, we’ve seen what might be the primary building blocks of memories lighting up in the brains of mice.
We have cells in our brains – and so do rodents – that keep track of our location and the distances we’ve travelled. These neurons are also known to fire in sequence when a rat is resting, as if the animal is mentally retracing its path – a process that probably helps memories form, says Rosa Cossart at the Institut de Neurobiologie de la Méditerranée in Marseille, France.
But without a way of mapping the activity of a large number of these individual neurons, the pattern that these replaying neurons form in the brain has been unclear. Researchers have suspected for decades that the cells might fire together in small groups, but nobody could really look at them, says Cossart.
To get a look, Cossart and her team added a fluorescent protein to the neurons of four mice. This protein fluoresces the most when calcium ions flood into a cell – a sign that a neuron is actively firing. The team used this fluorescence to map neuron activity much more widely than previous techniques, using implanted electrodes, have been able to do.
Observing the activity of more than 1000 neurons per mouse, the team watched what happened when mice walked on a treadmill or stood still.
As expected, when the mice were running, the neurons that trace how far the animal has travelled fired in a sequential pattern, keeping track.
These same cells also lit up while the mice were resting, but in a strange pattern. As they reflected on their memories, the neurons fired in the same sequence as they had when the animals were running, but much faster. And rather than firing in turn individually, they fired together in sequential blocks that corresponded to particular fragments of a mouse’s run.
“We’ve been able to image the individual building-blocks of memory,” Cossart says, each one reflecting a chunk of the original episode that the mouse experienced.
Continue Reading.


Today is World Anaesthesia Day! Here’s a look at the chemistry behind a range of anaesthetics. More info here and here.
-
 knightmare-of-luthia liked this · 8 years ago
knightmare-of-luthia liked this · 8 years ago -
 laboratoryretriever reblogged this · 8 years ago
laboratoryretriever reblogged this · 8 years ago -
 antimony-ammonites reblogged this · 9 years ago
antimony-ammonites reblogged this · 9 years ago -
 doublxb reblogged this · 9 years ago
doublxb reblogged this · 9 years ago -
 kruunk liked this · 9 years ago
kruunk liked this · 9 years ago -
 theunpopularbelief reblogged this · 9 years ago
theunpopularbelief reblogged this · 9 years ago -
 antiboyz-040 liked this · 9 years ago
antiboyz-040 liked this · 9 years ago -
 elsaofficefurniture liked this · 9 years ago
elsaofficefurniture liked this · 9 years ago -
 gebeine liked this · 9 years ago
gebeine liked this · 9 years ago -
 yoko-choko liked this · 9 years ago
yoko-choko liked this · 9 years ago -
 saraseb reblogged this · 9 years ago
saraseb reblogged this · 9 years ago -
 snapcrackl3-spock liked this · 9 years ago
snapcrackl3-spock liked this · 9 years ago -
 kind-hearted-rebel liked this · 9 years ago
kind-hearted-rebel liked this · 9 years ago -
 doctordearie reblogged this · 9 years ago
doctordearie reblogged this · 9 years ago -
 doctordearie liked this · 9 years ago
doctordearie liked this · 9 years ago -
 hedminreg liked this · 9 years ago
hedminreg liked this · 9 years ago -
 hammadichakouath liked this · 9 years ago
hammadichakouath liked this · 9 years ago -
 sloppy96 liked this · 9 years ago
sloppy96 liked this · 9 years ago -
 mimosa203 liked this · 9 years ago
mimosa203 liked this · 9 years ago -
 yeezynation8-blog liked this · 9 years ago
yeezynation8-blog liked this · 9 years ago -
 rfish342-blog liked this · 9 years ago
rfish342-blog liked this · 9 years ago -
 contradictiontonature reblogged this · 9 years ago
contradictiontonature reblogged this · 9 years ago -
 donniedamsel-blog reblogged this · 9 years ago
donniedamsel-blog reblogged this · 9 years ago -
 kcrump24 reblogged this · 9 years ago
kcrump24 reblogged this · 9 years ago -
 sloftycookies liked this · 9 years ago
sloftycookies liked this · 9 years ago -
 thebabeingray reblogged this · 9 years ago
thebabeingray reblogged this · 9 years ago -
 pumpkaboosworld reblogged this · 9 years ago
pumpkaboosworld reblogged this · 9 years ago -
 smekedandreket-blog liked this · 9 years ago
smekedandreket-blog liked this · 9 years ago -
 komikbookgeek reblogged this · 9 years ago
komikbookgeek reblogged this · 9 years ago -
 littlemuseums liked this · 9 years ago
littlemuseums liked this · 9 years ago -
 thejrtlikes liked this · 9 years ago
thejrtlikes liked this · 9 years ago -
 merhasstellyrhas reblogged this · 9 years ago
merhasstellyrhas reblogged this · 9 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts