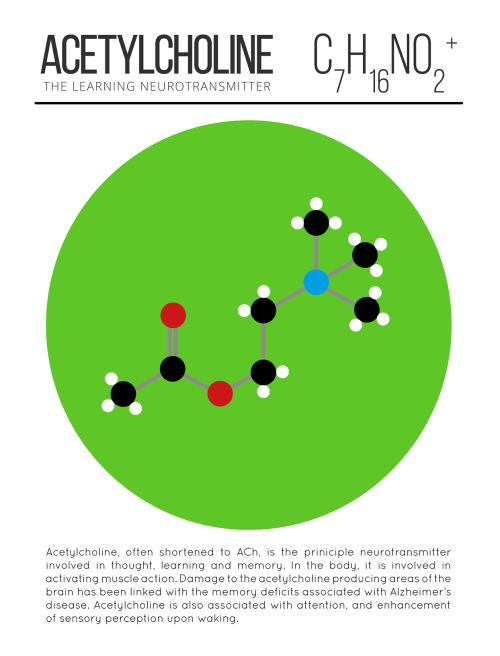
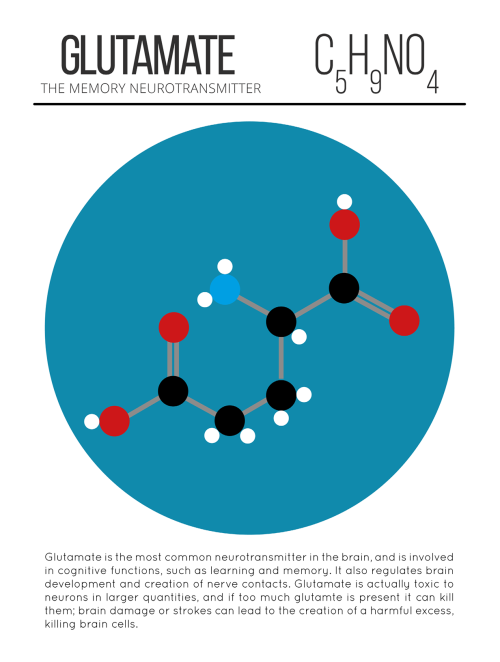
The Time Will Come When Diligent Research Over Long Periods Will Bring To Light Things Which Now Lie
The time will come when diligent research over long periods will bring to light things which now lie hidden. A single lifetime, even though entirely devoted to the sky, would not be enough for the investigation of so vast a subject… And so this knowledge will be unfolded through long successive ages. There will come a time when our descendants will be amazed that we did not know things that are so plain to them… Many discoveries are reserved for ages still to come, when memory of us will have been effaced. Our universe is a sorry little affair unless it has something for every age to investigate… Nature does not reveal her mysteries once and for all.
Seneca
(via scienceisbeauty)
More Posts from Contradictiontonature and Others
Checking Cancer At Its Origin..
In a first, the lab led by Leonard Zon at Boston Children’s Hospital has visualised the emergence of the primary melanoma cell in transgenic zebrafish that harbour the human oncogenic BRAFV600E mutation in melanocytes. This cancerous state is characterised in maturing fish by the formation of neural crest progenitors [NCPs], which are the predecessors of melanocytes and are only seen in the embryonic stage of healthy zebrafish.
The Zon lab placed the human mutated oncogene, BRAFV600E (a characteristic of benign human nevi/moles) under the control of a melanocyte-specific promoter and introduced it into the zebrafish. Generations of this transgenic fish were engineered such that they were also deficient in functional p53 (loss of function mutation). They used previous findings that in healthy zebrafish, a gene called crestin is expressed only in the embryonic NCPs and never throughout maturity, but is re-expressed selectively in melanomatous cells during adulthood. crestin was cloned adjacent to a reporter, enhanced green fluorescent protein [EGFP] for live imaging purposes.
The developmental phases of the fish, that were by now triple transgenic (for human BRAFV600E, p53 LOF and crestin:EGFP) were observed by live imaging; ~21 days after fertilisation, the expression of crestin:EGFP localised precisely to the (future) melanoma sites, and the very first triple-transgenic (individual) cells that went on to form larger masses of cells were also observed. To summarise, melanoma formation was observed in three stages: individual fluorescent cells, followed by these cells multiplying to form groups of <50 cells, and lastly these groups forming raised lesions. This consistently held true, with all 30 observed individual cells turning into 30 lesions. These results are illustrated in Figure 1.

Figure 1. In the top left box, a single cell is visualised as it multiplies into a group of melanoma cells (top right). The bottom images show the raised melanoma lesion as observed by the naked eye and by live imaging. The green fluorescence emitted from EGFP indicates that it is localised only to the melanoma (as is crestin expression), that is, it has not metastasised elsewhere.
These pre-cancerous cells were also shown to be self-sustaining and tumourigenic: when fish scales containing the mutant cells were transplanted to another part of the same fish (auto-transplant) or to another fish (allo-transplant) that was also exposed to radiation, the cells proliferated in the new site, as well as penetrated the hypodermis underneath (Figure 2).

Figure 2. The fluorescence indicates a single scale being auto-transplanted elsewhere on the same fish. As the days progress, the patch expands as well, and after day 33, the cells penetrate deeper into the hypodermis and thrive independently, and excising the transplanted scale proves futile.
Role of Transcription Factor sox10
sox10 is a master TF in NCP and its over-expression has been correlated with increased crestin expression, and accordingly, sox10 over expression in the transgenic melanocytes accelerated the melanoma onset. Following the logical train of thought that sox10 promotes melanoma progression, it was then targeted by CRISPR-Cas9 and inactivated in the transgenic cells. This resulted in a delayed onset of melanoma (180 days) compared to the controls (133 days). sox10 is also known to be expressed in most human melanoma cell lines. Moreover, the DNA element that acts as the binding site for Sox10 is also found in a hyper-acetylated [H3K27Ac], super-enhancer state. This is an epigenetic alteration and may prove a useful target in therapy (ex. HAT inhibitors).
Summary
The key finding clears up a hitherto ambiguous association between a reversion to stem/progenitor cell-like status and cancer: it indicates that the apparent devolution of a specialised cell to a primitive cellular state is not a consequence of cancer progression, but that it is an hallmark of pre-cancerous cells that may contribute to tumour progression. The rarity of melanoma formation among the mutant cells also suggests that the double mutant [BRAFV600E; p53 LOF] is not the only factor to influence the onset. Experimentally, crestin expression was a definitive prelude to formation of nevi which transformed into full-fledged raised melanomas in that spot.
This discovery has two chronological applications: first, of the many susceptible melanocytes harbouring the mutated oncogene, we can find out which are most likely to enter the melanoma state. Peaks in the expression profile of sox2, or a couple other TFs, dlx2 and tfap2, can prove to be a telltale pre-melanoma signature and thus be used in diagnosis. Secondly, by doing so, these can be better targeted early on before they’ve disseminated and become virtually untreatable.
Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351[6272]:aad2197–aad2197.

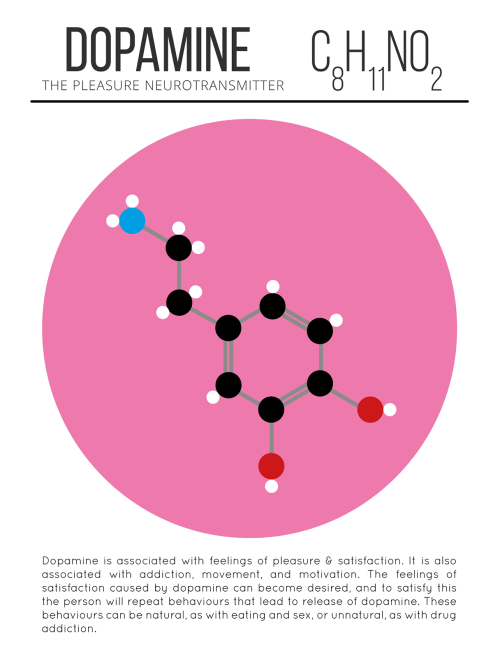
Happy Valentine’s Day! Hope everybody gets their share of dopamine and oxytocin today. #lovefeelings #scientificliteracy #braininlove #brainfeels

The 2016 Nobel Prize in Chemistry is awarded to Jean-Pierre Sauvage, Sir Fraser Stoddart, and Bernard Feringa for the design and production of molecular machines with controllable movements: bit.ly/NobelSci2016

WHAT??? Time to update those textbooks.
Did life begin on land rather than in the sea?
Stromatolites are round, multilayered mineral structures that range from the size of golf balls to weather balloons and represent the oldest evidence that there were living organisms on Earth 3.5 billion years ago.
Scientists who believed life began in the ocean thought these mineral formations had formed in shallow, salty seawater, just like living stromatolites in the World Heritage-listed area of Shark Bay, which is a two-day drive from the Pilbara.
But what Djokic discovered amid the strangling heat and blood-red rocks of the region was evidence that the stromatolites had not formed in salt water but instead in conditions more like the hot springs of Yellowstone.
The discovery pushed back the time for the emergence of microbial life on land by 580 million years and also bolstered a paradigm-shifting hypothesis laid out by UC Santa Cruz astrobiologists David Deamer and Bruce Damer: that life began, not in the sea, but on land.
Stromatolites.Credit: © Ints / Fotolia

Swarms of magnetic bacteria could be used to deliver drugs to tumors
Researchers funded in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have recently shown that magnetic bacteria are a promising vehicle for more efficiently delivering tumor-fighting drugs. They reported their results in the August 2016 issue of Nature Nanotechnology.
Ouajdi Felfoul, Mahmood Mohammadi, Samira Taherkhani, Dominic de Lanauze, Yong Zhong Xu, Dumitru Loghin, Sherief Essa, Sylwia Jancik, Daniel Houle, Michel Lafleur, Louis Gaboury, Maryam Tabrizian, Neila Kaou, Michael Atkin, Té Vuong, Gerald Batist, Nicole Beauchemin, Danuta Radzioch, Sylvain Martel. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nature Nanotechnology, 2016; DOI: 10.1038/nnano.2016.137
Illustration showing magnetic bacteria delivering drugs to a tumor. Credit: NanoRobotics Laboratory, Polytechnique Montreal
-
 striveforgreatnessss liked this · 6 years ago
striveforgreatnessss liked this · 6 years ago -
 nathaly-meow reblogged this · 6 years ago
nathaly-meow reblogged this · 6 years ago -
 nathaly-meow liked this · 6 years ago
nathaly-meow liked this · 6 years ago -
 dearkurisu liked this · 6 years ago
dearkurisu liked this · 6 years ago -
 moonlcver liked this · 6 years ago
moonlcver liked this · 6 years ago -
 bluearrejon reblogged this · 7 years ago
bluearrejon reblogged this · 7 years ago -
 ihaditall reblogged this · 8 years ago
ihaditall reblogged this · 8 years ago -
 luleyez liked this · 8 years ago
luleyez liked this · 8 years ago -
 luleyez reblogged this · 8 years ago
luleyez reblogged this · 8 years ago -
 inglewoodken reblogged this · 8 years ago
inglewoodken reblogged this · 8 years ago -
 cloudywithachanceofknighttime reblogged this · 8 years ago
cloudywithachanceofknighttime reblogged this · 8 years ago -
 aspiringdev liked this · 8 years ago
aspiringdev liked this · 8 years ago -
 midnightmayhemwithme reblogged this · 8 years ago
midnightmayhemwithme reblogged this · 8 years ago -
 midnightmayhemwithme liked this · 8 years ago
midnightmayhemwithme liked this · 8 years ago -
 scarlet-fire1918 liked this · 8 years ago
scarlet-fire1918 liked this · 8 years ago -
 jheanelashlee liked this · 8 years ago
jheanelashlee liked this · 8 years ago -
 --dopamine reblogged this · 8 years ago
--dopamine reblogged this · 8 years ago -
 hir0protagonist-blog liked this · 8 years ago
hir0protagonist-blog liked this · 8 years ago -
 heroandheroine1 liked this · 8 years ago
heroandheroine1 liked this · 8 years ago -
 d0 reblogged this · 8 years ago
d0 reblogged this · 8 years ago -
 unseelier reblogged this · 8 years ago
unseelier reblogged this · 8 years ago -
 drmarsvin reblogged this · 8 years ago
drmarsvin reblogged this · 8 years ago -
 microwave-coffee reblogged this · 8 years ago
microwave-coffee reblogged this · 8 years ago -
 playboktai reblogged this · 8 years ago
playboktai reblogged this · 8 years ago -
 iswearimnotacynic reblogged this · 8 years ago
iswearimnotacynic reblogged this · 8 years ago -
 iswearimnotacynic liked this · 8 years ago
iswearimnotacynic liked this · 8 years ago -
 papilliondepapier liked this · 8 years ago
papilliondepapier liked this · 8 years ago -
 derikjacobson reblogged this · 8 years ago
derikjacobson reblogged this · 8 years ago -
 guidethyperplexed liked this · 8 years ago
guidethyperplexed liked this · 8 years ago -
 vincent-the-narrator reblogged this · 8 years ago
vincent-the-narrator reblogged this · 8 years ago -
 theconglomeration reblogged this · 8 years ago
theconglomeration reblogged this · 8 years ago -
 theseeker1864 liked this · 8 years ago
theseeker1864 liked this · 8 years ago -
 polymorphiczooid liked this · 8 years ago
polymorphiczooid liked this · 8 years ago -
 thekateisalie liked this · 8 years ago
thekateisalie liked this · 8 years ago -
 panda-poes reblogged this · 8 years ago
panda-poes reblogged this · 8 years ago -
 cuteserialkiller liked this · 8 years ago
cuteserialkiller liked this · 8 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts