The Hubble Space Telescope Captured This Picture Of The Wispy Remains Of A Supernova Explosion. The Dust

The Hubble Space Telescope captured this picture of the wispy remains of a supernova explosion. The dust cloud in the upper center of the picture is the actual supernova remnant. The dense concentration of stars in the lower left is the outskirts of star cluster NGC 1850. Full resolution picture here. More info here. Credit: NASA, ESA, Y.-H. Chu (Academia Sinica, Taipei)
More Posts from Contradictiontonature and Others
Complement Pathways

Components of complement pathways of the immune system.
Classical Pathway: binds to the pathogen surface
C1 binds to phosphocholine on bacteria, which activates C1r to cleave C1s.
Activated C1s cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by C1s, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
MB-Lectin Pathway: uses mannin-binding lectin to bind to mannose-containing carbohydrates on the pathogen surface
Mannin-binding lectin (MBL) binds to the pathogen surface and activates MASP-2.
MASP-2 cleaves C4 to C4a and C4b.
C4b binds to the microbial surface and also binds C2.
C2 is cleaved to C2a and C2b by MASP-2, forming the C4bC2a complex.
The C4bC2a complex cleaves C3 to C3a and C3b.
C3b binds to the surface and causes opsonization.
Alternative Pathway: binds to the pathogen surface with spontaneously activated complement, amplifies C3b
C3b deposited by the C3 convertase binds to factor B.
Factor B is cleaved by factor D into Ba and Bb, forming the C3bBb complex.
The C3bBb complex cleaves C3 into C3a and C3b.
C3 spontaneously hydrolyzes to C3(H2O).
C3(H2O) binds to factor B, and factor D cleaves factor B.
Upon factor B cleavage, the C3(H2O)Bb complex is formed.
The C3(H2O)Bb complex cleaves C3 into C3a and C3b.
Factor B binds to C3b on the surface and is cleaved to Bb.
Checking Cancer At Its Origin..
In a first, the lab led by Leonard Zon at Boston Children’s Hospital has visualised the emergence of the primary melanoma cell in transgenic zebrafish that harbour the human oncogenic BRAFV600E mutation in melanocytes. This cancerous state is characterised in maturing fish by the formation of neural crest progenitors [NCPs], which are the predecessors of melanocytes and are only seen in the embryonic stage of healthy zebrafish.
The Zon lab placed the human mutated oncogene, BRAFV600E (a characteristic of benign human nevi/moles) under the control of a melanocyte-specific promoter and introduced it into the zebrafish. Generations of this transgenic fish were engineered such that they were also deficient in functional p53 (loss of function mutation). They used previous findings that in healthy zebrafish, a gene called crestin is expressed only in the embryonic NCPs and never throughout maturity, but is re-expressed selectively in melanomatous cells during adulthood. crestin was cloned adjacent to a reporter, enhanced green fluorescent protein [EGFP] for live imaging purposes.
The developmental phases of the fish, that were by now triple transgenic (for human BRAFV600E, p53 LOF and crestin:EGFP) were observed by live imaging; ~21 days after fertilisation, the expression of crestin:EGFP localised precisely to the (future) melanoma sites, and the very first triple-transgenic (individual) cells that went on to form larger masses of cells were also observed. To summarise, melanoma formation was observed in three stages: individual fluorescent cells, followed by these cells multiplying to form groups of <50 cells, and lastly these groups forming raised lesions. This consistently held true, with all 30 observed individual cells turning into 30 lesions. These results are illustrated in Figure 1.

Figure 1. In the top left box, a single cell is visualised as it multiplies into a group of melanoma cells (top right). The bottom images show the raised melanoma lesion as observed by the naked eye and by live imaging. The green fluorescence emitted from EGFP indicates that it is localised only to the melanoma (as is crestin expression), that is, it has not metastasised elsewhere.
These pre-cancerous cells were also shown to be self-sustaining and tumourigenic: when fish scales containing the mutant cells were transplanted to another part of the same fish (auto-transplant) or to another fish (allo-transplant) that was also exposed to radiation, the cells proliferated in the new site, as well as penetrated the hypodermis underneath (Figure 2).

Figure 2. The fluorescence indicates a single scale being auto-transplanted elsewhere on the same fish. As the days progress, the patch expands as well, and after day 33, the cells penetrate deeper into the hypodermis and thrive independently, and excising the transplanted scale proves futile.
Role of Transcription Factor sox10
sox10 is a master TF in NCP and its over-expression has been correlated with increased crestin expression, and accordingly, sox10 over expression in the transgenic melanocytes accelerated the melanoma onset. Following the logical train of thought that sox10 promotes melanoma progression, it was then targeted by CRISPR-Cas9 and inactivated in the transgenic cells. This resulted in a delayed onset of melanoma (180 days) compared to the controls (133 days). sox10 is also known to be expressed in most human melanoma cell lines. Moreover, the DNA element that acts as the binding site for Sox10 is also found in a hyper-acetylated [H3K27Ac], super-enhancer state. This is an epigenetic alteration and may prove a useful target in therapy (ex. HAT inhibitors).
Summary
The key finding clears up a hitherto ambiguous association between a reversion to stem/progenitor cell-like status and cancer: it indicates that the apparent devolution of a specialised cell to a primitive cellular state is not a consequence of cancer progression, but that it is an hallmark of pre-cancerous cells that may contribute to tumour progression. The rarity of melanoma formation among the mutant cells also suggests that the double mutant [BRAFV600E; p53 LOF] is not the only factor to influence the onset. Experimentally, crestin expression was a definitive prelude to formation of nevi which transformed into full-fledged raised melanomas in that spot.
This discovery has two chronological applications: first, of the many susceptible melanocytes harbouring the mutated oncogene, we can find out which are most likely to enter the melanoma state. Peaks in the expression profile of sox2, or a couple other TFs, dlx2 and tfap2, can prove to be a telltale pre-melanoma signature and thus be used in diagnosis. Secondly, by doing so, these can be better targeted early on before they’ve disseminated and become virtually untreatable.
Kaufman CK, Mosimann C, Fan ZP, Yang S, Thomas AJ, Ablain J, et al. A zebrafish melanoma model reveals emergence of neural crest identity during melanoma initiation. Science. 2016;351[6272]:aad2197–aad2197.
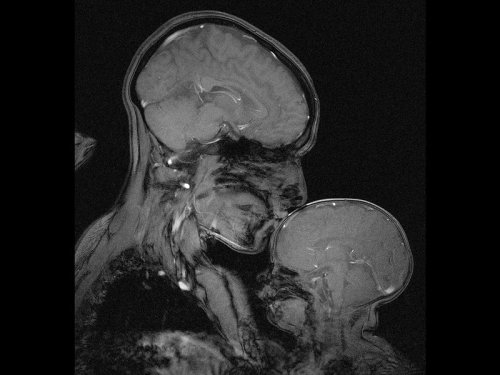
Neuroscientist captures an MRI of a mother and child
Professor Rebecca Saxe (MIT) has taken the first ever MR image of a mother and child.
“This picture is an MR image of a mother and a child that I made in my lab at MIT. You might see it as sweet and touching… an image of universal love. We can’t see clothes or hairstyles or even skin colour. From what we do see, the biology and the brains, this could be any mother and child or even father and child at any time and place in history; having an experience that any human can recognise.
Or you might see it as disturbing, a reminder that our human bodies are much too fragile as houses for ourselves. MRI’s are usually medical images and often bad news. Each white spot in that picture is a blood vessel that could clog, each tiny fold of those brains could harbour a tumour. The baby’s brain maybe looks particularly vulnerable pressed against the soft thin shell of its skull.
I see those things, universal emotions and frightening fragility but I also see one of the most amazing transformations in biology.”
Quotes have been taken from a TEDx talk given by Professor Saxe explaining the story behind the above picture.


COLORS OF CHEMISTRY
The bright colors of chemistry fascinate people of all ages. Hriday Bhattacharjee, a Ph.D. student in the lab of Jens Mueller at the University of Saskatchewan, assembled this showcase from compounds he prepared as well as from some synthesized by the undergraduate students he teaches. Organometallic and inorganic chemistry—the study of molecules like these that involve metal atoms—is especially colorful.
The table below the picture indicates the chemicals seen in the photo.
Submitted by Hriday Bhattacharjee
Do science. Take pictures. Win money: Enter our photo contest.

Memory Competition
Most of the brain contains cells that no longer divide and renew. However, the dentate gyrus, nestled within the memory-forming centre of the brain (the hippocampus) is one of the few sites where new cells continue to form throughout life. As a person ages, there is an ever-increasing struggle for these new dentate gyrus neurons (coloured pink) to integrate with existing older neurons (green) because the latter already has well-established connections. This may be why learning and memorisation becomes more difficult as a person gets older. Scientists have now found that by temporarily reducing the number of dendritic spines – branches of neurons that form connections with other neurons – in the mature cells, the new cells have a better chance of functionally integrating. Indeed, in live mice, briefly eliminating dendritic spines boosted the number of integrated new neurons, which rejuvenated the hippocampus and improved the animals’ memory precision.
Written by Ruth Williams
Image courtesy of Kathleen McAvoy
Center for Regenerative Medicine, Massachusetts General Hospital, Boston, MA, USA
Copyright held by original authors
Research published in Neuron, September 2016
You can also follow BPoD on Twitter and Facebook

Poison in the Brain: Toxic Structures in Neuronal Nuclei of Alzheimer’s Patients
Spherical structures in the nucleus of nerve cells, so-called nuclear spheres, are suspected to trigger Alzheimer’s disease. A team headed by Dr Thorsten Müller from the research group Cell Signaling in Neurodegeneration has for the very first time demonstrated the presence of the presumably toxic protein aggregates in the human brain. The researchers from Ruhr-Universität Bochum have published their article in the journal Neurobiology of Aging.
The team compared brain samples from Alzheimer’s patients with those of the healthy individuals in the same age group. The result: in the samples taken from Alzheimer’s patients, the number of nuclear spheres was much higher than in those taken from healthy study participants.
Moreover, the group from Bochum analysed in what way nuclear spheres are generated. It was demonstrated in experiments with cell cultures that the amyloid precursor protein (APP) plays a crucial role in this process. APP has long been associated with Alzheimer’s disease. The researchers observed that nuclear sphere generation preferably takes place, if the amyloid precursor protein carries no phosphate group in a specific amino acid. An APP cleavage product, moreover, is contained in the nuclear spheres.
“Extensive nuclear sphere generation in the human Alzheimer’s brain” by Katharina Kolbe, Hassan Bukhari, Christina Loosse, Gregor Leonhardt, Annika Glotzbach, Magdalena Pawlas, Katharina Hess, Carsten Theiss, and Thorsten Müller in Neurobiology of Aging. Published online August 18 2016 doi:10.1016/j.neurobiolaging.2016.08.016

This massive virus with its own immune system could hold the future of medicine
French researchers think they’ve found a giant virus big enough to house its own virus-killing devices using a system like CRISPR, and it could be a completely new form of life.
Called a mimivirus, it was first found growing in amoebae in a water tower. At four times the size of a typical virus, you can even see it under a light microscope
When the mimivirus encounters another virus, it stores some of the invader’s genetic material. That way, when it encounters the same kind of virus again, the MIMIVIRE system goes into gene-editing berserker mode, finding the key genes of the virus and cutting them to inert oblivion. This could have major applications.
Follow @the-future-now

Happy Valentine’s Day! Hope everybody gets their share of dopamine and oxytocin today. #lovefeelings #scientificliteracy #braininlove #brainfeels

One of the largest icebergs ever recorded, packing about a trillion tons of ice or enough to fill up two Lake Eries, has just split off from Antarctica, in a much anticipated, though not celebrated, calving event.
A section of the Larsen C ice shelf with an area of 2,240 square miles (5,800 square kilometers) finally broke away some time between July 10 and today (July 12), scientists with the U.K.-based MIDAS Project, an Antarctic research group, reported today.
Continue Reading.
-
 lies-of-our-lives reblogged this · 2 years ago
lies-of-our-lives reblogged this · 2 years ago -
 lies-of-our-lives liked this · 2 years ago
lies-of-our-lives liked this · 2 years ago -
 tainbow07 liked this · 5 years ago
tainbow07 liked this · 5 years ago -
 theinconstantmoon reblogged this · 6 years ago
theinconstantmoon reblogged this · 6 years ago -
 orbeliosis liked this · 6 years ago
orbeliosis liked this · 6 years ago -
 not-a-bit-tamed reblogged this · 6 years ago
not-a-bit-tamed reblogged this · 6 years ago -
 not-a-bit-tamed liked this · 6 years ago
not-a-bit-tamed liked this · 6 years ago -
 bxrnadxttx liked this · 6 years ago
bxrnadxttx liked this · 6 years ago -
 star-stuff-in-the-cosmos reblogged this · 6 years ago
star-stuff-in-the-cosmos reblogged this · 6 years ago -
 bluejaywvy liked this · 6 years ago
bluejaywvy liked this · 6 years ago -
 cityscapeskeletons liked this · 6 years ago
cityscapeskeletons liked this · 6 years ago -
 modernvalkyrie reblogged this · 6 years ago
modernvalkyrie reblogged this · 6 years ago -
 x--monpechemignon reblogged this · 6 years ago
x--monpechemignon reblogged this · 6 years ago -
 imgayandexhausted liked this · 6 years ago
imgayandexhausted liked this · 6 years ago -
 ushijimbo reblogged this · 6 years ago
ushijimbo reblogged this · 6 years ago -
 youroptimisticblackhole reblogged this · 6 years ago
youroptimisticblackhole reblogged this · 6 years ago -
 w0jt liked this · 6 years ago
w0jt liked this · 6 years ago -
 th0ca liked this · 6 years ago
th0ca liked this · 6 years ago -
 subtle-chaos liked this · 6 years ago
subtle-chaos liked this · 6 years ago -
 subtle-chaos reblogged this · 6 years ago
subtle-chaos reblogged this · 6 years ago -
 valdinhx liked this · 6 years ago
valdinhx liked this · 6 years ago -
 redhousehead reblogged this · 6 years ago
redhousehead reblogged this · 6 years ago -
 redhousehead liked this · 6 years ago
redhousehead liked this · 6 years ago -
 youfinexx reblogged this · 6 years ago
youfinexx reblogged this · 6 years ago -
 coletassoft reblogged this · 6 years ago
coletassoft reblogged this · 6 years ago -
 rauchendym liked this · 6 years ago
rauchendym liked this · 6 years ago -
 dropthedaggers liked this · 6 years ago
dropthedaggers liked this · 6 years ago -
 i-kin-these-bitches liked this · 6 years ago
i-kin-these-bitches liked this · 6 years ago -
 ignition07 liked this · 6 years ago
ignition07 liked this · 6 years ago -
 life-is-a-jokesstuff liked this · 6 years ago
life-is-a-jokesstuff liked this · 6 years ago -
 jplca liked this · 6 years ago
jplca liked this · 6 years ago -
 rrrrrrmmmmmmlllll liked this · 6 years ago
rrrrrrmmmmmmlllll liked this · 6 years ago -
 lilyblack31 liked this · 6 years ago
lilyblack31 liked this · 6 years ago -
 paranoid4prof3t liked this · 6 years ago
paranoid4prof3t liked this · 6 years ago -
 sevenvik liked this · 6 years ago
sevenvik liked this · 6 years ago -
 panda-poes reblogged this · 6 years ago
panda-poes reblogged this · 6 years ago -
 anonymousastronomer liked this · 6 years ago
anonymousastronomer liked this · 6 years ago -
 raccoon-wizard liked this · 6 years ago
raccoon-wizard liked this · 6 years ago -
 galileostelescope liked this · 6 years ago
galileostelescope liked this · 6 years ago -
 rain7nites liked this · 6 years ago
rain7nites liked this · 6 years ago -
 faithless34-blog liked this · 6 years ago
faithless34-blog liked this · 6 years ago -
 talldrinkawater66 reblogged this · 6 years ago
talldrinkawater66 reblogged this · 6 years ago -
 navarroartandimage liked this · 6 years ago
navarroartandimage liked this · 6 years ago -
 governor--megatron reblogged this · 6 years ago
governor--megatron reblogged this · 6 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts