We-are-all-paranoid - Microbe Nerd Alert

More Posts from We-are-all-paranoid and Others

Plz reblog, I need to know I'm not alone in this.



‘aBiogenesis’ Reimagines the Primordial Soup Theory in a Mesmerizing Animation by Markos Kay
How SARS-CoV-2 Hijacks Human Cells to Evade Immune System

Researchers at University of California San Diego School of Medicine have discovered one way in which SARS-CoV-2, the coronavirus that causes COVID-19, hijacks human cell machinery to blunt the immune response, allowing it to establish infection, replicate and cause disease.
In short, the virus’ genome gets tagged with a special marker by a human enzyme that tells the immune system to stand down, while at the same time ramping up production of the surface proteins that SARS-CoV-2 uses as a “doorknob” to enter cells.
The study, published April 22, 2021 in Cell Reports, helps lay the groundwork for new anti-viral immunotherapies — treatments that work by boosting a patient’s immune system, rather than directly killing the virus.
“It’s very smart of this virus to use host machinery to simultaneously go into stealth mode and get inside more cells,” said Tariq Rana, PhD, professor and chief of the Division of Genetics in the Department of Pediatrics at UC San Diego School of Medicine and Moores Cancer Center. “The more we know about how the virus establishes itself in the body, the better equipped we are to disrupt it.”
In human cells, genes (DNA) are transcribed into RNA, which is then translated into proteins, the molecules that make up the majority of cells. But it’s not always so straightforward. Cells can chemically modify RNA to influence protein production. One of these modifications is the addition of methyl groups to adenosine, one of the building blocks that make up RNA. Known as N6-methyladenosine (m6A), this modification is common in humans and other organisms, including viruses.
In contrast to humans, the entire genomes of some viruses, including SARS-CoV-2, are made up of RNA instead of DNA. And rather than carry around the machinery to translate that into proteins, the coronavirus gets human cells to do the work.
Rana and his team previously discovered that m6A plays an important role in HIV and Zika virus infections. In their latest study, the researchers discovered that the human enzyme METTL3 adds methyl groups to introduce m6A in SARS-CoV-2’s RNA. That modification prevents the virus’ RNA from triggering inflammatory molecules known as cytokines. To the team’s surprise, METTL3’s activity also led to increased expression of pro-viral genes — those that encode proteins needed for SARS-CoV-2 replication and survival, such as ACE2, the cell surface receptor that the virus uses to enter human cells.
“It remains to be seen why our cells help the virus out like this,” Rana said.
“How SARS-CoV-2 Hijacks Human Cells to Evade Immune System“
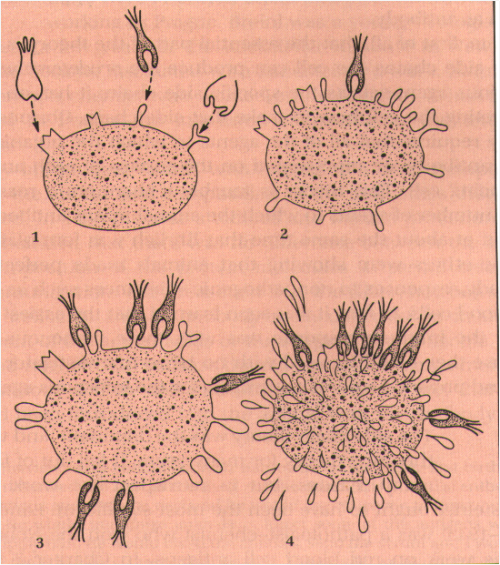
Antibodies are the secreted form of B-lymphocyte receptors and are a part of adaptive immunity, but how are these proteins formed?
Above is a diagram illustrating Paul Ehlrich’s Side Chain Theory of Antibody Formation. Ehlrich proposed that immunoglobulin molecules, a fundamental component of adaptive immunity, served as membrane bound proteins that bound to particular threats, similarly to the former “key in lock” view of enzymes in catalyzing biological reactions. Ehrlich also suggested that the action of binding a pathogenic molecule to the receptor would generate a signal to stimulate the production of more receptors of the same specificity. These “side chains” that were added on would then break off from the cell surface and become what we call antibodies.
We now know, however, that soluble immunoglobulin receptors are specially manufactured to be secreted as antibody, rather than just “breaking off” of the lymphocyte, even though they have the same specificity as their membrane-bound counterparts.

look at these gorgeous bronchial cartilage cells!
Fun fact: my dad, after being a surgeon for 25 years, no longer has fingerprints. The sponge he uses to wash his hands several times a day is so harsh that it’s rubbed off his fingerprints throughout the years. Sometimes he can’t get into our building because the biometric uses a fingerprint scanner 😭

Reminder to Click for Palestine today!
Click for the other causes as well if you can!
-
 thoraperson liked this · 7 months ago
thoraperson liked this · 7 months ago -
 anomalous-skink reblogged this · 7 months ago
anomalous-skink reblogged this · 7 months ago -
 ramattrasbigball2 liked this · 8 months ago
ramattrasbigball2 liked this · 8 months ago -
 meeelosh reblogged this · 8 months ago
meeelosh reblogged this · 8 months ago -
 gabbythegaysquid liked this · 8 months ago
gabbythegaysquid liked this · 8 months ago -
 mokomonkey liked this · 8 months ago
mokomonkey liked this · 8 months ago -
 megmelodia reblogged this · 8 months ago
megmelodia reblogged this · 8 months ago -
 flinthillsmonster reblogged this · 8 months ago
flinthillsmonster reblogged this · 8 months ago -
 pantestudines reblogged this · 8 months ago
pantestudines reblogged this · 8 months ago -
 pantestudines liked this · 8 months ago
pantestudines liked this · 8 months ago -
 junoniadoesart liked this · 8 months ago
junoniadoesart liked this · 8 months ago -
 beelper-owo liked this · 8 months ago
beelper-owo liked this · 8 months ago -
 urabrask-the-hidden reblogged this · 8 months ago
urabrask-the-hidden reblogged this · 8 months ago -
 urabrask-the-hidden liked this · 8 months ago
urabrask-the-hidden liked this · 8 months ago -
 pieisnotreal reblogged this · 8 months ago
pieisnotreal reblogged this · 8 months ago -
 nastyboot liked this · 8 months ago
nastyboot liked this · 8 months ago -
 scoobysnaxforcrev liked this · 8 months ago
scoobysnaxforcrev liked this · 8 months ago -
 mattdvm reblogged this · 8 months ago
mattdvm reblogged this · 8 months ago -
 mattdvm liked this · 8 months ago
mattdvm liked this · 8 months ago -
 the-timeline-owner reblogged this · 8 months ago
the-timeline-owner reblogged this · 8 months ago -
 the-timeline-owner liked this · 8 months ago
the-timeline-owner liked this · 8 months ago -
 anomalous-skink liked this · 8 months ago
anomalous-skink liked this · 8 months ago -
 circularcheez-it liked this · 8 months ago
circularcheez-it liked this · 8 months ago -
 spacedragonstar liked this · 8 months ago
spacedragonstar liked this · 8 months ago -
 ghostinthestatic liked this · 8 months ago
ghostinthestatic liked this · 8 months ago -
 ash-trashcan reblogged this · 8 months ago
ash-trashcan reblogged this · 8 months ago -
 hellagator reblogged this · 8 months ago
hellagator reblogged this · 8 months ago -
 cocolacola liked this · 8 months ago
cocolacola liked this · 8 months ago -
 kork-likes-bacon liked this · 8 months ago
kork-likes-bacon liked this · 8 months ago -
 sewer-dweller liked this · 8 months ago
sewer-dweller liked this · 8 months ago -
 dampfnudeldove liked this · 8 months ago
dampfnudeldove liked this · 8 months ago -
 sleepysnucker liked this · 8 months ago
sleepysnucker liked this · 8 months ago -
 paleontologylife reblogged this · 8 months ago
paleontologylife reblogged this · 8 months ago -
 paleontologylife liked this · 8 months ago
paleontologylife liked this · 8 months ago -
 we-are-all-paranoid reblogged this · 8 months ago
we-are-all-paranoid reblogged this · 8 months ago
