Inside - Vadim Sadovski








Inside - Vadim Sadovski
More Posts from T-sci-eng and Others
What is Liquid Penetrant Testing?
Liquid penetrant testing (LT) is a non-destructive testing technique utilized to detect defects or discontinuities (such as cracks) on the surface of any type of non-porous material such as metal, plastics or ceramics. Liquid penetrant testing (also known as dye penetrant testing or penetrant testing) is primarily utilized in the industrial sector to test metal materials such as oil & gas pipelines and various metal machinery components to prevent failures or accidents. Some of the many defects that can be detected using this process include fatigue cracks, hairline cracks and porosity. A number of industries utilize liquid penetrant testing, including petrochemical, aerospace, engineering, automotive and many more.
Although liquid penetrant testing is the least technologically advanced method of non-destructive testing (with the others being ultrasonic testing, magnetic particle testing and radiography) – it is still widely used. That’s because liquid penetrant testing has the advantages of being low in cost, versatile and easy to perform. In fact, liquid penetrant testing requires very little training when compared to the other three main forms of non-destructive testing.
So exactly how does liquid penetrant testing work? The material to be tested must first be cleaned – usually using a simple spray cleaner that can be easily wiped off with a cloth or rag. A liquid penetrant solution is then applied to the surface of the material being tested using a simple aerosol spray from a can. The liquid is then left to soak for a predetermined length of time – and will eventually seep into or be drawn into any cracks or defects within the material being tested. After the appropriate amount of “soak time” has passed, the technician wipes the liquid penetrant off of the test object. A developer is then applied to the entire area being tested. The developer is usually a dry white powder such as chalk that is suspended in liquid and sprayed on in aerosol form. The developer then acts to draw out any liquid that may have seeped into a defect – giving a highly visible, colored indication on the surface of the test object.
Liquid penetrant testing relies solely on visual inspection – making the color contrast between the object being tested and the colored indication that reveals defects of utmost importance. For this reason, many technicians utilize fluorescents. This process is the same as conventional liquid penetrant testing, with the exception that a fluorescent penetrant is utilized and then the test object is viewed under ultraviolet light in a darkened environment. The result is that any defects present will glow brightly under the UV light – making visual inspection much easier.
Aside from the obvious advantages of being inexpensive and easy to use, liquid penetrant testing is also popular because of its versatility. In most cases, nothing more than three aerosol cans – cleaner, penetrant and developer – and a few cloths or rags are needed. This allows technicians to easily maneuver into tight spaces such as boilers or high places where ladders are required – easily completing testing in locations where other non-destructive testing techniques are difficult or impossible. For these reasons, liquid penetrant testing continues to be a viable and popular non-destructive testing method.
Tech Service Products is a stocking distributor of industrial supplies and non-destructive testing products such as liquid penetrant testing products.
Puritans, Goths, avant-garde artists, hell-raising poets and fashion icon Coco Chanel all saw something special in it. Now black, that most enigmatic of colours, has become even darker and more mysterious.
A British company has produced a “strange, alien” material so black that it absorbs all but 0.035 per cent of visual light, setting a new world record. To stare at the “super black” coating made of carbon nanotubes – each 10,000 times thinner than a human hair – is an odd experience. It is so dark that the human eye cannot understand what it is seeing. Shapes and contours are lost, leaving nothing but an apparent abyss.

The dihydrogen monoxide hoax involves calling water by the unfamiliar chemical name “dihydrogen monoxide” (DHMO), and listing some of water’s effects in an alarming manner, such as the fact that it accelerates corrosion and can cause severe burns. The hoax often calls for dihydrogen monoxide to be regulated, labeled as hazardous, or banned. It illustrates how the lack of scientific literacy and an exaggerated analysis can lead to misplaced fears.
The hoax gained renewed popularity in the late 1990s when a 14-year-old student collected anti-DHMO petitions for a science project about gullibility. The story has since been used in science education to encourage critical thinking and avoid the appeal to nature.
Forty-three students favored banning DHMO, six were undecided, and only one correctly recognized that ‘dihydrogen monoxide’ is actually plain old water.
Here’s the information he gave the students:
Dihydrogen monoxide is colorless, odorless, tasteless, and kills uncounted thousands of people every year. Most of these deaths are caused by accidental inhalation of DHMO, but the dangers of dihydrogen monoxide do not end there. Prolonged exposure to its solid form causes severe tissue damage. Symptoms of DHMO ingestion can include excessive sweating and urination, and possibly a bloated feeling, nausea, vomiting and body electrolyte imbalance. For those who have become dependent, DHMO withdrawal means certain death.
Dihydrogen monoxide:
is also known as hydroxl acid, and is the major component of acid rain.
contributes to the “greenhouse effect.”
may cause severe burns.
contributes to the erosion of our natural landscape.
accelerates corrosion and rusting of many metals.
may cause electrical failures and decreased effectiveness of automobile brakes.
has been found in excised tumors of terminal cancer patients.
Contamination is reaching epidemic proportions!
Quantities of dihydrogen monoxide have been found in almost every stream, lake, and reservoir in America today. But the pollution is global, and the contaminant has even been found in Antarctic ice. DHMO has caused millions of dollars of property damage in the midwest, and recently California.
Despite the danger, dihydrogen monoxide is often used:
as an industrial solvent and coolant.
in nuclear power plants.
in the production of styrofoam.
as a fire retardant.
in many forms of cruel animal research.
in the distribution of pesticides. Even after washing, produce remains contaminated by this chemical.
as an additive in certain “junk-foods” and other food products.
Companies dump waste DHMO into rivers and the ocean, and nothing can be done to stop them because this practice is still legal. The impact on wildlife is extreme, and we cannot afford to ignore it any longer!
The American government has refused to ban the production, distribution, or use of this damaging chemical due to its “importance to the economic health of this nation.” In fact, the navy and other military organizations are conducting experiments with DHMO, and designing multi-billion dollar devices to control and utilize it during warfare situations. Hundreds of military research facilities receive tons of it through a highly sophisticated underground distribution network. Many store large quantities for later use.
Source: [x]
Click HERE for more facts

Vantablack absorbs 99% of light and is the darkest material ever made.
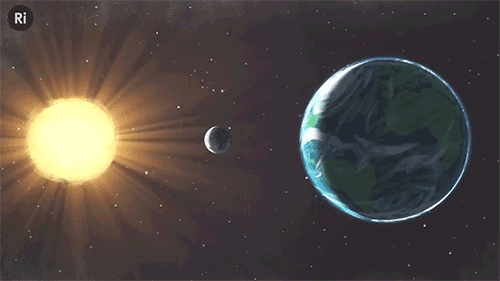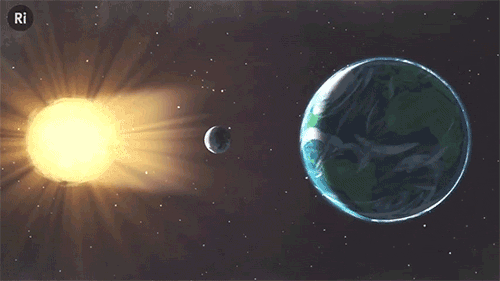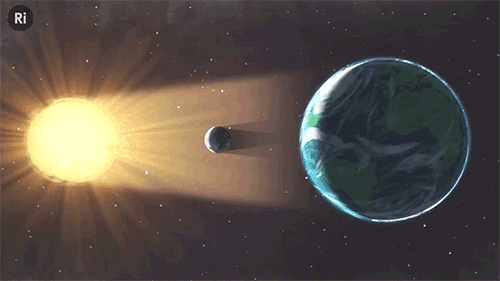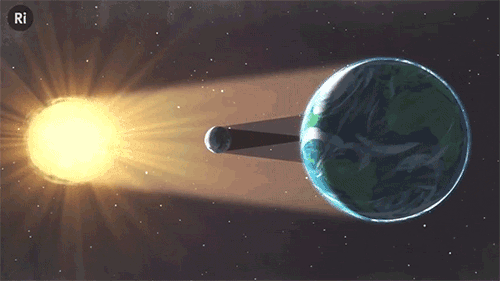
What have eclipses ever done for science? Quite a lot, actually!
The first measurement of the width of the Atlantic ocean in the 16th Century



When British settlers arrived in Virginia in the US, they weren’t sure how far across the globe they’d gone. They recorded the local time of a total eclipse of the moon - which is seen all across the night-time side of the planet. Their colleagues in London did the same, and when the travellers returned they could figure out the five hour time difference.
Edmond Halley discovered that the moon is moving away from the Earth


Halley realised you could back-calculate when previous eclipses would have occurred. But he noticed a mismatch between his predictions and the history books. The reason, he discovered, what that he was assuming the moon stayed the same distance from the Earth. It is actually getting further at about the rate your fingernails grow. And that means that one day (in a few million years, that is), the moon will be too far away to create any more total solar eclipses.
In 1919 a solar eclipse proved Einstein’s theory of relativity

Einstein’s theory predicted that the sun’s gravity should bend the light of nearby stars, meaning that in theory we should be able to see stars that are hidden just behind the sun. However, sunlight always blocks our view of these stars, and it was only during a solar eclipse that there was a short window to see if hidden stars were visible, as predicted. Astronomer Arthur Eddington travelled to West Africa and took photos that proved Einstein right.
Scientists still use solar eclipses today
It’s very hard to study the sun’s corona - a tenuous hot gas, which just one millionth of the light intensity of the sun. The shapes and lines of the corona show the nature of the sun’s magnetic field, and are only visible to study during an eclipse. NASA are also using this opportunity to help create the first thermal map of Mercury!
Want to know more? Watch our full video.

New superglue allows for bonding stretchable hydrogels
A team of researchers at Johannes Kepler University Linz has developed a new type of glue that can be used to bond hydrogels to other hard or soft objects. In their paper published on the open-access site Science Advances, the group explains their development process, the structure of the glue, how it works and in what ways.
Hydrogels, as the name suggests, are materials made mainly out of water. They are typically rubbery and are often elastic. Many of them have been developed to allow for the creation of materials that are more like those found in living creatures. Some examples include soft contact lenses, soft bone replacement in the vertebrae and even jelly-like robots. But one thing that has been holding back more advanced applications is the inability to glue or bond hydrogels with other objects in ways that allow for bending or stretching, or even for attaching well to hard objects. In this new effort, the researchers report they have developed a glue that solves this problem.
Read more.
Hi I made this app called PhysicsPedia. It contains all the theory and formulas of high school and college physics. Please have a look. https://play.google.com/store/apps/details?id=com.pp.nikit.phyprac2
Editor’s Note:
Thank you for your submission android-nikit and we did check the app out and it is really interesting and useful. If you are in high school, we recommend you give this one a try!
Flat tires could eventually be a thing of the past. Michelin has unveiled the concept for a 3-D printed, airless tire.
follow @the-future-now
Centrifugal force and seat belts

The basic concept of a seatbelt is to protect you in an automobile collision by holding you in your seat. This prevents you from flying forward and colliding with the dashboard or windshield.
How do you do that ?
Many common seat belts design have something known as a centrifugal clutch. This arrangement has a weight attached to the end of a spool
When the spool rotates at a low speed, the weight is held through spring action and is allowed to spin freely.

But you must have noticed that if you try to pull the seat belt faster then it kinda gets stuck.
This is because as you rotate the spool faster, centrifugal force causes the weight to be pushed out and that stops the spool from rotating further.

This adds tension to your seat belt and holds you to your seat at the time of a crash.
Have a great day!
* Other seatbelt mechanisms
** Seatbelt physics



Why most metals are silver (but copper and gold aren’t)
If we want to understand what gives a metal its color we first need to understand a little bit about the definition of a metal. Metals are materials that experience metallic bonding - wherein the atoms are so close that there is a veritable “sea of electrons” in the substance. (This is also what makes metals conductors, but that’s another story). Basically each atom donates an electron or two that is free to flow throughout the material, unattached to any particular nucleus.
This proximity leads to an overlap in the allowed energy levels of electrons (shown in the lower left hand image above); basically the higher empty electronic levels are so close to the uppermost filled levels (also called the Fermi level) that they form an essentially continuous band of allowed energies.
Now, backtracking a little bit, color in a substance is caused when a material doesn’t absorb a particular wavelength of light. Because of the empty energy levels mentioned above, metals generally can absorb all wavelengths of light in the visible spectrum. This implies that a metal should look black, except that the excited electron can immediately fall back to the state that it came from, emitting exactly the same energy, causing a flat piece of metal to appear reflective. Thus, the reason why most metals are silver. (Also, the flatter a metal, the more reflective, thanks to diffuse vs. specular reflection).
For a few select metals, like copper and gold, the absorption and emission of photons are noticeably dependent on wavelength across the visible part of the spectrum. The graph in the lower right image above shows the reflectance of aluminum, silver, and gold, including wavelengths in the infrared and ultraviolet. Aluminum is pretty reflective overall, and silver is highly reflective in the visible region (about 400 to 700 nm), but gold clearly absorbs wavelengths about 500 nm or below. Thus, it most strongly reflects yellow, giving it its characteristic appearance.
Sources: (first image), 2 (second image), 3 (third image), 4
-
 lunarian-queen reblogged this · 1 week ago
lunarian-queen reblogged this · 1 week ago -
 fuzzypilled liked this · 1 month ago
fuzzypilled liked this · 1 month ago -
 angeluscore reblogged this · 1 month ago
angeluscore reblogged this · 1 month ago -
 helldidntwantme reblogged this · 1 month ago
helldidntwantme reblogged this · 1 month ago -
 mira-xx liked this · 1 month ago
mira-xx liked this · 1 month ago -
 sushgdgd liked this · 3 months ago
sushgdgd liked this · 3 months ago -
 pipper075 liked this · 4 months ago
pipper075 liked this · 4 months ago -
 wawa-7 reblogged this · 4 months ago
wawa-7 reblogged this · 4 months ago -
 wawa-7 liked this · 4 months ago
wawa-7 liked this · 4 months ago -
 annabananabread96 liked this · 5 months ago
annabananabread96 liked this · 5 months ago -
 mrsattila reblogged this · 5 months ago
mrsattila reblogged this · 5 months ago -
 pink-soul-diary liked this · 5 months ago
pink-soul-diary liked this · 5 months ago -
 pineconeeaterr liked this · 6 months ago
pineconeeaterr liked this · 6 months ago -
 yaad1g liked this · 6 months ago
yaad1g liked this · 6 months ago -
 hiken-no-heli liked this · 6 months ago
hiken-no-heli liked this · 6 months ago -
 vengeg liked this · 7 months ago
vengeg liked this · 7 months ago -
 06-09-06 liked this · 8 months ago
06-09-06 liked this · 8 months ago -
 soengineergarden liked this · 9 months ago
soengineergarden liked this · 9 months ago -
 s2m2nk liked this · 9 months ago
s2m2nk liked this · 9 months ago -
 whiterabbityogini reblogged this · 10 months ago
whiterabbityogini reblogged this · 10 months ago -
 leda-timeandspace liked this · 1 year ago
leda-timeandspace liked this · 1 year ago -
 voracidadelunar reblogged this · 1 year ago
voracidadelunar reblogged this · 1 year ago -
 voracidadelunar liked this · 1 year ago
voracidadelunar liked this · 1 year ago -
 tightywhitieshemabearmark liked this · 1 year ago
tightywhitieshemabearmark liked this · 1 year ago -
 legalassassino liked this · 1 year ago
legalassassino liked this · 1 year ago -
 galacticaries reblogged this · 1 year ago
galacticaries reblogged this · 1 year ago -
 galacticaries liked this · 1 year ago
galacticaries liked this · 1 year ago -
 famestoshiro reblogged this · 1 year ago
famestoshiro reblogged this · 1 year ago -
 famestoshiro liked this · 1 year ago
famestoshiro liked this · 1 year ago -
 ourcoralstar reblogged this · 1 year ago
ourcoralstar reblogged this · 1 year ago -
 ifihadhomework reblogged this · 1 year ago
ifihadhomework reblogged this · 1 year ago -
 erykahbadont244 reblogged this · 1 year ago
erykahbadont244 reblogged this · 1 year ago -
 veelie666 liked this · 1 year ago
veelie666 liked this · 1 year ago -
 bonesofyesterdays liked this · 1 year ago
bonesofyesterdays liked this · 1 year ago -
 impredecible-e liked this · 1 year ago
impredecible-e liked this · 1 year ago -
 eyonsen liked this · 1 year ago
eyonsen liked this · 1 year ago -
 cmlotus liked this · 1 year ago
cmlotus liked this · 1 year ago -
 redjaybird liked this · 1 year ago
redjaybird liked this · 1 year ago -
 erhogonfasync liked this · 1 year ago
erhogonfasync liked this · 1 year ago -
 bigborger reblogged this · 1 year ago
bigborger reblogged this · 1 year ago -
 bigborger liked this · 1 year ago
bigborger liked this · 1 year ago -
 horse-shit reblogged this · 1 year ago
horse-shit reblogged this · 1 year ago -
 i-dont-exis liked this · 1 year ago
i-dont-exis liked this · 1 year ago
