Largest Batch Of Earth-size, Habitable Zone Planets
Largest Batch of Earth-size, Habitable Zone Planets
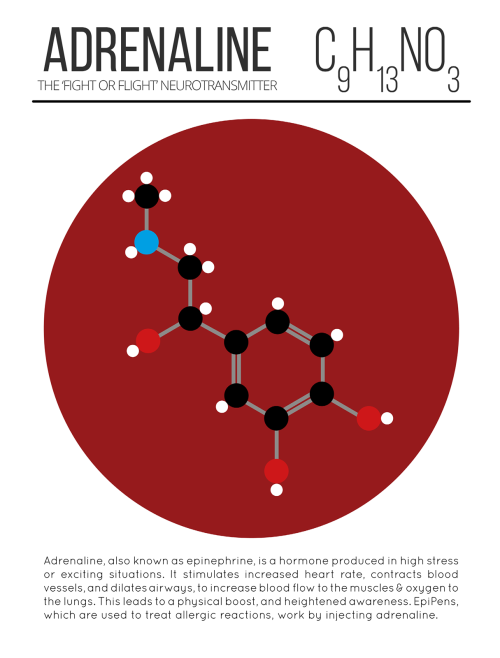
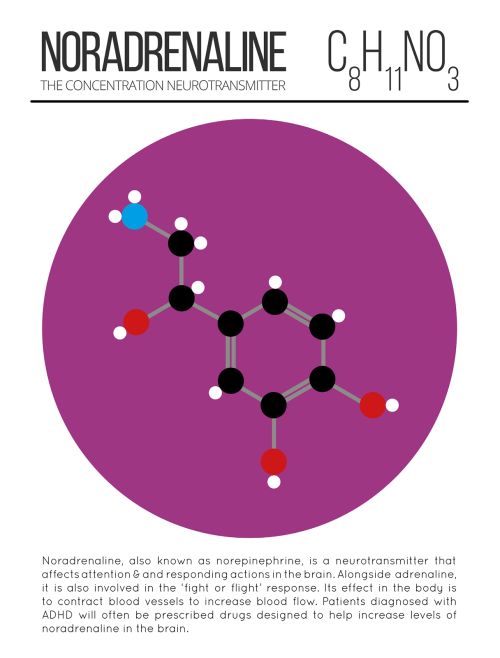
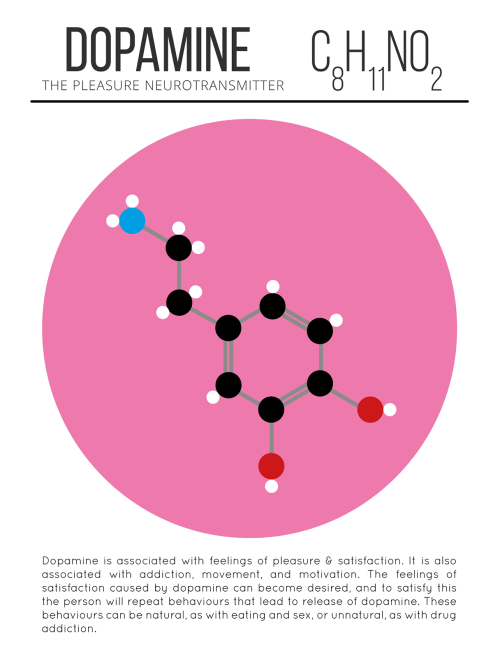
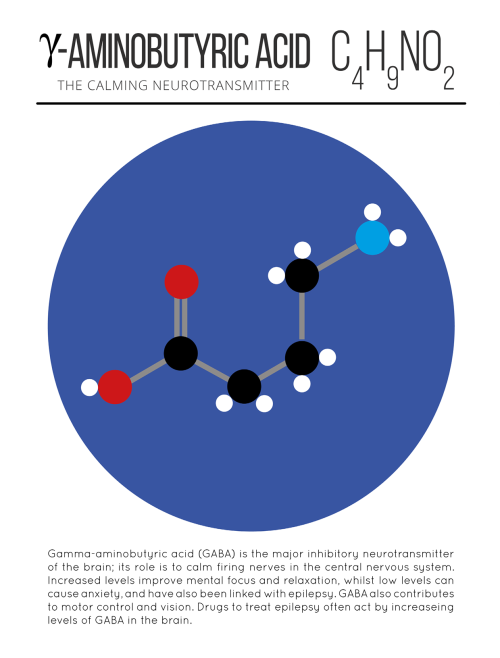
Our Spitzer Space Telescope has revealed the first known system of seven Earth-size planets around a single star. Three of these planets are firmly located in an area called the habitable zone, where liquid water is most likely to exist on a rocky planet.

This exoplanet system is called TRAPPIST-1, named for The Transiting Planets and Planetesimals Small Telescope (TRAPPIST) in Chile. In May 2016, researchers using TRAPPIST announced they had discovered three planets in the system.

Assisted by several ground-based telescopes, Spitzer confirmed the existence of two of these planets and discovered five additional ones, increasing the number of known planets in the system to seven.

This is the FIRST time three terrestrial planets have been found in the habitable zone of a star, and this is the FIRST time we have been able to measure both the masses and the radius for habitable zone Earth-sized planets.
All of these seven planets could have liquid water, key to life as we know it, under the right atmospheric conditions, but the chances are highest with the three in the habitable zone.

At about 40 light-years (235 trillion miles) from Earth, the system of planets is relatively close to us, in the constellation Aquarius. Because they are located outside of our solar system, these planets are scientifically known as exoplanets. To clarify, exoplanets are planets outside our solar system that orbit a sun-like star.

In this animation, you can see the planets orbiting the star, with the green area representing the famous habitable zone, defined as the range of distance to the star for which an Earth-like planet is the most likely to harbor abundant liquid water on its surface. Planets e, f and g fall in the habitable zone of the star.
Using Spitzer data, the team precisely measured the sizes of the seven planets and developed first estimates of the masses of six of them. The mass of the seventh and farthest exoplanet has not yet been estimated.

For comparison…if our sun was the size of a basketball, the TRAPPIST-1 star would be the size of a golf ball.
Based on their densities, all of the TRAPPIST-1 planets are likely to be rocky. Further observations will not only help determine whether they are rich in water, but also possibly reveal whether any could have liquid water on their surfaces.
The sun at the center of this system is classified as an ultra-cool dwarf and is so cool that liquid water could survive on planets orbiting very close to it, closer than is possible on planets in our solar system. All seven of the TRAPPIST-1 planetary orbits are closer to their host star than Mercury is to our sun.

The planets also are very close to each other. How close? Well, if a person was standing on one of the planet’s surface, they could gaze up and potentially see geological features or clouds of neighboring worlds, which would sometimes appear larger than the moon in Earth’s sky.

The planets may also be tidally-locked to their star, which means the same side of the planet is always facing the star, therefore each side is either perpetual day or night. This could mean they have weather patterns totally unlike those on Earth, such as strong wind blowing from the day side to the night side, and extreme temperature changes.

Because most TRAPPIST-1 planets are likely to be rocky, and they are very close to one another, scientists view the Galilean moons of Jupiter – lo, Europa, Callisto, Ganymede – as good comparisons in our solar system. All of these moons are also tidally locked to Jupiter. The TRAPPIST-1 star is only slightly wider than Jupiter, yet much warmer.
How Did the Spitzer Space Telescope Detect this System?
Spitzer, an infrared telescope that trails Earth as it orbits the sun, was well-suited for studying TRAPPIST-1 because the star glows brightest in infrared light, whose wavelengths are longer than the eye can see. Spitzer is uniquely positioned in its orbit to observe enough crossing (aka transits) of the planets in front of the host star to reveal the complex architecture of the system.

Every time a planet passes by, or transits, a star, it blocks out some light. Spitzer measured the dips in light and based on how big the dip, you can determine the size of the planet. The timing of the transits tells you how long it takes for the planet to orbit the star.

The TRAPPIST-1 system provides one of the best opportunities in the next decade to study the atmospheres around Earth-size planets. Spitzer, Hubble and Kepler will help astronomers plan for follow-up studies using our upcoming James Webb Space Telescope, launching in 2018. With much greater sensitivity, Webb will be able to detect the chemical fingerprints of water, methane, oxygen, ozone and other components of a planet’s atmosphere.
At 40 light-years away, humans won’t be visiting this system in person anytime soon…that said…this poster can help us imagine what it would be like:

Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com
More Posts from Contradictiontonature and Others
The gut bacteria inside 1000-year-old mummies from the Inca Empire are resistant to most of today’s antibiotics, even though we only discovered these drugs within the last 100 years.
“At first we were very surprised,” Tasha Santiago-Rodriguez of California Polytechnic State University in San Louis Opisbo, told the Annual Meeting of the American Society for Microbiology last month.
Her team studied the DNA within the guts of three Incan mummies dating back to between the 10th and 14thcenturies and six mummified people from Italy, from between the 15th and 18th centuries. They found an array of genes that have the potential to resist almost all modern antibiotics, including penicillin, vancomycin and tetracycline.
These ancient genes were largely in microbes whose resistance is problematic today, including Enteroccocus bacteria that can infect wounds and cause urinary tract infections. But they found that many other species, including some harmless ones, carried some of these resistant genes too.
Enterococcus enigma
“When you think about it, almost all these antibiotics are naturally produced, so it makes sense to find antibiotic genes as well,” says Santiago-Rodriguez.
Their finding shows that genes that can confer resistance to antibiotics were relatively widespread hundreds of years before Alexander Fleming discovered penicillin in 1928. “It’s ridiculous to think evolution of antibiotic resistance began when penicillin was discovered,” said team-member Raul Cano, also at California Polytechnic State University, at the meeting while discussing the findings. “It’s been going on for 2 billion years.”
These genes existed long before antibiotics became common, but it is our overuse of these drugs in both people and livestock that caused the superbug resistance to explode worldwide, said Cano.
“This is exciting data,” says Adam Roberts, who studies antibiotic resistance genes at University College London. While it is already well known that antibiotic resistance occurred naturally before people started using antibiotics, this study shows that resistance genes were already within the human gut long before we started using these drugs, he says.
“It begs the question of what was selecting for these genes at this time? Was it the natural production of antibiotics by other bacteria, or were there other, as yet unknown forces at play?” asks Roberts.

Finally got the pure Nile Red in solution, just need to evaporate to get the pure dye.
Interesting fact: Nile Red is a solvatochromic dye. What does this mean? Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents. Also its emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with with different color depending on the solvent what it’s dissolved in.
In this case it was dissolved in dichloromethane.


The Red Hot Debate about Transmissible Alzheimer’s
In the 25 years that John Collinge has studied neurology, he has seen hundreds of human brains. But the ones he was looking at under the microscope in January 2015 were like nothing he had seen before.
He and his team of pathologists were examining the autopsied brains of four people who had once received injections of growth hormone derived from human cadavers. It turned out that some of the preparations were contaminated with a misfolded protein—a prion—that causes a rare and deadly condition called Creutzfeldt–Jakob disease (CJD), and all four had died in their 40s or 50s as a result. But for Collinge, the reason that these brains looked extraordinary was not the damage wrought by prion disease; it was that they were scarred in another way. “It was very clear that something was there beyond what you’d expect,” he says. The brains were spotted with the whitish plaques typical of people with Alzheimer’s disease. They looked, in other words, like young people with an old person’s disease.
For Collinge, this led to a worrying conclusion: that the plaques might have been transmitted, alongside the prions, in the injections of growth hormone—the first evidence that Alzheimer’s could be transmitted from one person to another. If true, that could have far-reaching implications: the possibility that ‘seeds’ of the amyloid-β protein involved in Alzheimer’s could be transferred during other procedures in which fluid or tissues from one person are introduced into another, such as blood transfusions, organ transplants and other common medical procedures.
Collinge felt a duty to inform the public quickly. And that’s what he did, publishing the study inNature in September, to headlines around the world. “Can you CATCH Alzheimer’s?” asked Britain’s Daily Mail, about the “potentially explosive new study”. Collinge has been careful to temper the alarm. “Our study does not mean that Alzheimer’s is actually contagious,” he stresses. Carers won’t catch it on the job, nor family members, however close. “But it raises concern that some medical procedures could be inadvertently transferring amyloid-β seeds.”
Since then, the headlines have died away, but the academic work and discussion have taken off. Could seeds of amyloid-β proteins really be transmitted and, if so, are they harmless or do they cause disease? And could seeds of other related diseases involving misfolded proteins be transmitted in a similar way? In the past decade or so, evidence has been mounting for a controversial theory that rogue proteins, known collectively as amyloids and associated with diverse neurodegenerative diseases—from Alzheimer’s to Parkinson’s and Huntington's—might share some properties of prions, including their transmissibility. Collinge’s data bolstered that theory.
Urgent though these questions are, it could take years to find answers. The paper by Collinge and his colleagues has sparked a worldwide hunt for similar amyloid pathology in autopsied brains, and a small study published in January 2016 revealed a handful of related cases. Researchers are also trying to work out what the putative amyloid seeds look like, and whether different 'strains’ of amyloids exist that are particularly damaging.
Some researchers say that it is much too early to be alarmed. They point out that the number of patients in Collinge’s study was tiny, that none had displayed symptoms of Alzheimer’s disease before their death and that another toxic protein called tau also seems to be required to cause the condition. “We have to remember that there is no conclusive evidence that seeds of amyloids can transmit actual disease or that amyloids spread in the brain in a prion-like way,” says Pierluigi Nicotera, scientific director of the German Centre for Neurodegenerative Diseases in Bonn. “There may be other biological explanations.”
Right now, there are few solid answers, but plenty of concerns. The sceptics worry that they might one day find themselves working under tight biosecurity regulations to handle proteins that they view as relatively innocuous. Others feel that the dangers may have been underestimated, and that scientists have a duty to investigate this as quickly as they can. “In my opinion, all amyloids should be considered dangerous until proven safe,” says prion and amyloid researcher Adriano Aguzzi at the University Hospital Zurich in Switzerland.
DANGEROUS FOLDS
A few decades ago, it was almost inconceivable that a protein, which has no genetic material or any other obvious way to self-replicate, could cause infectious disease. But that changed in 1982, when Stanley Prusiner, now at the University of California, San Francisco, introduced evidence for disease-causing prions, coining the term from the words 'proteinacious’ and 'infectious’. Prusiner showed that prion proteins (PrP) exist in a normal cellular form, and in a misfolded infectious form. The misfolded form causes the normal protein to also misfold, creating a cascade that overwhelms and kills cells. It cause animal brains to turn into a spongy mess in scrapie, a disease of sheep, and in bovine spongiform encephalopathy (BSE or 'mad cow disease’), as well as in human prion diseases such as CJD.
Prusiner and others also investigated how prions could spread. They showed that injecting brain extracts containing infectious prions into healthy animals seeds disease. These prions can be so aggressive that in some cases, simply eating infected brains is sufficient to transmit disease. For example, many cases of variant CJD (vCJD) are now thought to have arisen in the United Kingdom in the 1990s after people ate meat from cattle that were infected with BSE.
Since then, scientists have come to appreciate that many proteins associated with neurodegenerative diseases—including amyloid-β and tau in Alzheimer’s disease and α-synuclein in Parkinson’s disease—misfold catastrophically. Structural biologists call the entire family of misfolded proteins (including PrP) amyloids. Amyloid-β clumps into whitish plaques, tau forms ribbons called tangles and α-synuclein creates fibrous deposits called inclusions.
A decade ago, these similarities prompted neuroscientist Mathias Jucker at the University of Tübingen in Germany to test whether injecting brain extracts containing misfolded amyloid-β into mice could seed an abnormal build-up of amyloid in the animals’ brains. He found that it could, and that it also worked if he injected amyloids into the muscles. “We saw no reason not to believe that if amyloid seeds entered the human brain, they would also cause amyloid pathology in the same way,” says Jucker.
This didn’t cause alarm at the time, because it wasn’t clear how an amyloid seed from the brain of someone with Alzheimer’s could be transferred into another person’s body and find its way to their brain. To investigate that, what was needed was a group of people who had been injected with material from another person, and the opportunity to examine their brains in great detail, preferably when they were still relatively young and before they might have spontaneously developed early signs of Alzheimer’s.
The CJD brains provided just that opportunity. Between 1958 and 1985, around 30,000 people worldwide received injections of growth hormone derived from the adrenal glands of cadavers to treat growth problems. Some of the preparations were contaminated with the prion that causes CJD. Like all prion diseases, CJD has a very long incubation period, but once it gets going it rages through the brain, destroying all tissue in its wake and typically killing people from their late 40s onwards. According to 2012 statistics, 226 people around the world have died from CJD as a result of prion-contaminated growth-hormone preparations.
Collinge had not set out to find a link with Alzheimer's—it emerged as part of routine work at the National Prion Clinic in London, which he heads, and where around 70% of all people in the United Kingdom who die from prion-related causes are now autopsied. The clinic routinely looks for signs of all amyloid proteins in these brains to distinguish prion disease from other conditions. It was thanks to this routine work that the cluster of unusual cases emerged of people who had clearly died of CJD, but who also had obvious signs of amyloid pathology in their grey matter and cerebral blood vessels.
As soon as he saw these brains, Collinge knew that he could get into stormy waters. Keen to strike a balance between warning of a possible public-health risk and causing unwarranted panic, he sketched a carefully worded press release that would go out from the National Prion Centre and set up hotlines for people who had been treated with growth hormone in the past. But no panic occurred: apart from one or two overwrought headlines, the news stories were fairly measured, he says. Only around ten people called the hotlines.
For scientists, however, the paper was a red flag. “As soon as the paper came out we realized the health implications and started collecting slides and paraffin blocks from patients,” says Jiri Safar, director of the National Prion Disease Pathology Surveillance Center at Case Western Reserve University in Cleveland, Ohio. Like other pathologists in countries where people had died of CJD associated with medical procedures, he rushed to check the centre’s archives of autopsied brains to see if any of them contained the ominous amyloid deposits.
The answers are not yet in. Safar says that it has not proved easy to trace brain samples in the United States, but that he is working to do so with the National Institutes of Health and the Centers for Disease Control and Prevention (CDC) in Atlanta, Georgia. Charles Duyckaerts at the Pitié-Salpêtrière Hospital in Paris, France, has now examined brain tissues from around 24 patients and is likely to report the results later this year.
A further 228 cases of CJD were caused by transplantation of prion-contaminated dura mater—the membrane surrounding the brain and spinal cord—prepared from cadavers around the world. Dura-mater preparations were regularly used in brain surgery as repair patches until the late 1990s. For the study published in January, Herbert Budka at the National Prion Diseases Reference Center at University Hospital Zurich and his colleagues examined the brains of seven such patients from Switzerland and Austria, and found that five had amyloid deposits in grey matter and blood vessels. In Japan, dementia researcher Masahito Yamada at Kanazawa University is making his way through a large number of such autopsy specimens and says that the 16 brains he has examined so far show signs of unusually high levels of amyloid deposition in cerebral blood vessels.
Yet such case studies can only ever provide circumstantial evidence that seeds of amyloid-β were transferred during the treatments. And they cannot entirely rule out the possibility that the treatments themselves—or the patients’ original medical conditions—caused the amyloid pathology. More-conclusive evidence would come from checking whether the original growth hormone and dura-mater preparations contained infectious amyloid seeds, by injecting them into animals and seeing whether this triggers disease. Most of these preparations, however, have long since disappeared. Collinge has access to some original samples of growth hormone stored by the UK Department of Health, and he is planning to analyse them for the presence of amyloid seeds and then inject them into mice. That work will take a couple of years to complete, he says.
SEEDS OF DOUBT
There is another hitch: no one knows for sure what size and shape the amyloid seeds might be. Jucker is hunting for them in an unusual source of human brain tissue that has nothing to do with CJD. A team in Bonn has collected frozen samples from more than 700 people with epilepsy who were operated on over the past 25 years to remove tissue that was driving their seizures. “It is the best source of fresh human brain tissue available at the moment,” says Jucker, who plans to scrutinize it carefully under the microscope for anything that might resemble tiny clumps or seeds of amyloid-β. The team also has records of the patients’ cognitive skills, such as language and memory skills, before and at regular intervals after the operations. This should allow Jucker’s team to correlate the presence of any amyloid-β seeds it finds with changes in the cognitive function of individual patients over time.
Scientists have shown that tau and α-synuclein can also seed pathological features in mice. In two studies, from 2012, scientists injected fibrils of α-synuclein into the brains of mice already engineered to develop some of the characteristics of Parkinson’s disease. This triggered the early onset of some of the signs and symptoms of Parkinson’s, and eventually killed the animals. A third study showed that similar injections into normal mice caused some of the neurodegeneration typical of Parkinson’s disease and the mice became less agile. In humans, α-synuclein would not necessarily turn out to be equally aggressive—mouse models of neurodegenerative diseases do not mimic human disease very closely—but scientists are taking the possibility seriously.
If the transmissibility hypothesis proves true, the implications could be severe. Amyloids stick like glue to metal surgical instruments, and normal sterilization does not remove them, so amyloid seeds might possibly be transferred during surgery. The seeds might sit in the body for years or decades before spreading into plaques, and perhaps enabling the other pathological changes needed to induce Alzheimer’s disease. Having amyloid plaques in cerebral blood vessels could be dangerous in another way, because they increase the risk that the vessel walls might break, leading to small strokes.
But if common medical procedures really increased the risk of neurodegenerative disorders, then wouldn’t that already have been detected? Not necessarily, says epidemiologist Roy Anderson at Imperial College London. “The proper epidemiological studies have not been done yet,” he says. They require very large and carefully curated databases of people with Alzheimer’s disease, which include information about the development of symptoms and autopsy data. He and his team are now studying the handful of reliable databases that exist to tease out a signal that might associate medical procedures with Alzheimer’s progression. The number of patients currently available may turn out to be too small to draw conclusions, he says, but a more definitive answer could emerge as the databases grow.
Faced with so much uncertainty, some researchers and public-health agencies have adopted a wait-and-see approach. “We are right at the beginning of this story,” says Nicotera, “and if there is one message to come out right now it is that we need more work to see if this is a relevant mechanism.” The CDC and the European Centre for Disease Prevention and Control in Solna, Sweden, say that they are keeping a cautious eye on the issue.
If further research does confirm that common neurodegenerative diseases are transmissible, what then? One immediate priority would be rigorous sterilization procedures for medical and surgical instruments that would destroy amyloids, in the way that extremely high temperatures and harsh chemicals destroy prions. Aguzzi says that funding agencies should put out calls now to researchers to develop cheap and simple sterilization methods. “It’s not very sexy science, but it is urgently needed,” he says. He also worries about the safety of researchers working with amyloids—particularly α-synuclein. “I have nightmares that someone in my lab may catch Parkinson’s,” he says. “While the story is in flux, our first duty is to protect lab workers.”
STRAIN SEEKERS
The similarities between prions and other amyloids is throwing open other avenues of research. Prions can exist as distinct strains—proteins that have the same sequence of amino acids but misfold in different ways and have distinct biological behaviours, much as different strains of a pathogenic virus can be aggressive or weak. The outbreak of vCJD in the United Kingdom in the 1990s was traced to BSE-contaminated meat because the prion strain was the same in both.
Over the past few years, research in animals has shown that different strains of amyloid-β and α-synuclein exist. And a landmark paper in 2013 reported that strains of amyloid-β with different 3D structures were associated with different disease progression in two people with Alzheimer’s. Structural biologist Robert Tycko, who led the work at the National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, Maryland, is now looking at many more brain samples from such patients.
Knowing the structures of pathological forms of amyloid seeds should help to design small molecules that bind to them and stop them doing damage, says biophysicist Ronald Melki at the Paris-Saclay Institute of Neuroscience, who works on α-synuclein strains. His lab is designing small peptides that target the seeds and mimic regions of 'chaperone’ molecules, which usually bind to proteins and help them to fold correctly. Melki’s small peptides mimic these binding regions, sticking to the amyloid proteins to stop them from aggregating further.
In the research community, much of the agitation in response to Collinge’s paper boils down to semantics. Some scientists do not like to use the word 'prion’ in connection with the amyloids associated with common neurodegenerative diseases, or to describe any of their properties as 'prion-like'—because of its connotation of infectious, deadly disease. “The public has this perception of the word 'prion’,” says Alzheimer’s researcher Brad Hyman at Harvard Medical School in Boston, Massachusetts, and this matters, even if their ideas are wrong. “One of my patients told me that she wasn’t getting any hugs any more from her husband who had read about the case in the media—that made me sad,” he says.
Others, however, feel that it is helpful to consider prions and other amyloids as being part of a single spectrum of conditions involving proteins that misfold and misbehave. It means that researchers studying prion diseases and neurodegenerative diseases, who until recently had considered their disciplines to be separate, now find themselves tackling shared questions.
Both fields are wary of raising premature alarm, even though they wonder what the future will bring. Jucker, only half-jokingly, says he could imagine a future in which people would go into hospital every ten years or so and get the amyloid seeds cleared out of their brains with antibodies. “You’d be good then to go for another decade.”
Image 1 Credit: ©iStock.com
Image 2 Credit: Juan Gaertner/Shutterstock
Source: Scientific American (By Alison Abbott, Nature magazine)
Steve Gentleman, a neuropathologist, demonstrates the process of brain dissection and preservation for research.

The multiverse might not be madness after all.
Alternate realities, parallel dimensions, and multiple universes. Whatever you call it, the notion of other versions of existence is one of the most popular tropes in science fiction. In some other universe, you’re not reading this sentence but skydiving. In another, you’re nothing but a cockroach. In yet another, not only is life impossible, but atoms don’t even exist.
In recent years, though, such seemingly crazy ideas have shifted from fantasy and speculation toward bona fide science. Even among physicists, the multiverse has gone mainstream.
Theoretically, infinite universes might stretch beyond our own, like endless bubbles in a sea of boiling water. Each bubble has its own laws of physics, and although we may never visit or even see another bubble, some physicists say growing evidence is making the multiverse increasingly plausible—and even probable. Learn more here.

Quote by #rosalindfranklin How do you make science a part of your life? What are you doing to fight for scientific literacy? More quotes and questions in my #ilovescience journal. #womeninscience #scientificliteracy




Archbishop Ussher’s chronology was taken as gospel in the Western world. Until we turned to another book to find the age of the earth, the one that was written in the rocks themselves.
One of the smoothest, most beautiful color changes I’ve ever seen.
The reaction is methoxymethyl deprotection of one of my agonists with concentrated HCl in acetonitrile as my solvent. The color change doesn’t happen in THF!
-
 nightmare-xlife reblogged this · 6 months ago
nightmare-xlife reblogged this · 6 months ago -
 nightmare-xlife liked this · 6 months ago
nightmare-xlife liked this · 6 months ago -
 wakayume liked this · 9 months ago
wakayume liked this · 9 months ago -
 l-nothinghere-l liked this · 1 year ago
l-nothinghere-l liked this · 1 year ago -
 kid-crayon liked this · 1 year ago
kid-crayon liked this · 1 year ago -
 lemaquillage liked this · 1 year ago
lemaquillage liked this · 1 year ago -
 imgudahevi liked this · 1 year ago
imgudahevi liked this · 1 year ago -
 sunbtidirag liked this · 1 year ago
sunbtidirag liked this · 1 year ago -
 mounworkprodan liked this · 1 year ago
mounworkprodan liked this · 1 year ago -
 bigjerkart liked this · 1 year ago
bigjerkart liked this · 1 year ago -
 luminoussphereofplasma reblogged this · 1 year ago
luminoussphereofplasma reblogged this · 1 year ago -
 evenica liked this · 1 year ago
evenica liked this · 1 year ago -
 iwillhaveamoonbase liked this · 2 years ago
iwillhaveamoonbase liked this · 2 years ago -
 damagedman liked this · 2 years ago
damagedman liked this · 2 years ago -
 ridazzle reblogged this · 2 years ago
ridazzle reblogged this · 2 years ago -
 ridazzle liked this · 2 years ago
ridazzle liked this · 2 years ago -
 antagonisttendencies reblogged this · 2 years ago
antagonisttendencies reblogged this · 2 years ago -
 pulchriate liked this · 2 years ago
pulchriate liked this · 2 years ago -
 g1itchb0y-advanced reblogged this · 2 years ago
g1itchb0y-advanced reblogged this · 2 years ago -
 g1itchb0y-advanced liked this · 2 years ago
g1itchb0y-advanced liked this · 2 years ago -
 usummonedme liked this · 2 years ago
usummonedme liked this · 2 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts