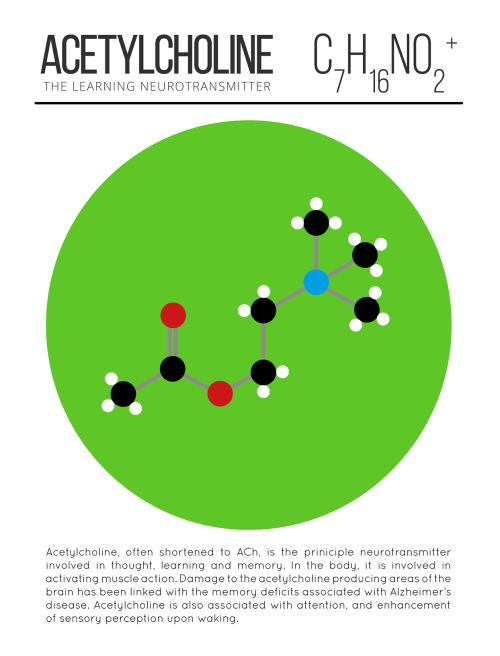
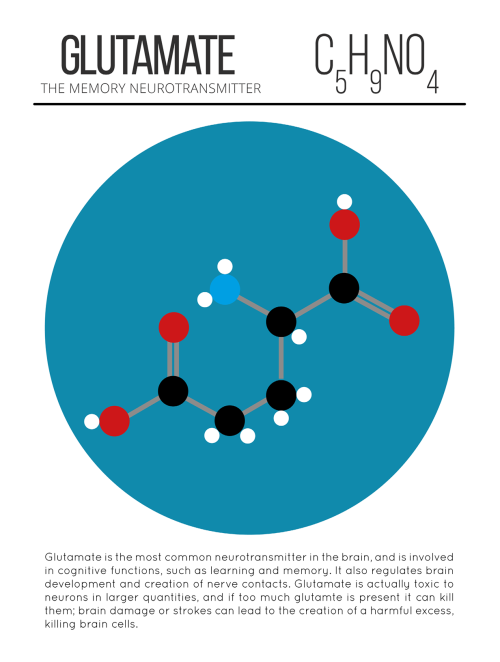
The Brilliant Colors Of A Soap Film Reveal The Fluid’s Thickness, Thanks To A Process Known As Thin
The brilliant colors of a soap film reveal the fluid’s thickness, thanks to a process known as thin film interference. The twisting flow of the film depends on many influences: gravity pulls down on the liquid and tends to make it drain away; evaporation steals fluid from the film; local air currents can push or pull the film; and the variation in the concentration of molecules – specifically the surfactants that stabilize the film – will change the local surface tension, causing flow via the Marangoni effect. Together these and other effects create the dancing turbulence captured above. (Video credit: A. Filipowicz)
More Posts from Contradictiontonature and Others

R.I.P. Vera Rubin; 1928-2016.
She never did win the Nobel prize for her discovery.

This year’s Halloween special wraps up the chemistry behind making a mummy: http://wp.me/p4aPLT-26m

Antibiotic resistance has been called one of the biggest public health threats of our time. There is a pressing need for new and novel antibiotics to combat the rise in antibiotic-resistant bacteria worldwide.
Researchers from Florida International University’s Herbert Wertheim College of Medicine are part of an international team that has discovered a new broad-spectrum antibiotic that contains arsenic. The study, published in Nature’s Communication Biology, is a collaboration between Barry P. Rosen, Masafumi Yoshinaga, Venkadesh Sarkarai Nadar and others from the Department of Cellular Biology and Pharmacology, and Satoru Ishikawa and Masato Kuramata from the Institute for Agro-Environmental Sciences, NARO in Japan.
“The antibiotic, arsinothricin or AST, is a natural product made by soil bacteria and is effective against many types of bacteria, which is what broad-spectrum means,” said Rosen, co-senior author of the study published in the Nature journal, Communications Biology. “Arsinothricin is the first and only known natural arsenic-containing antibiotic, and we have great hopes for it.”
Although it contains arsenic, researchers say they tested AST toxicity on human blood cells and reported that “it doesn’t kill human cells in tissue culture.”
Continue Reading.
Thumpety thump thump thumpety thump thump look at Kinesin go
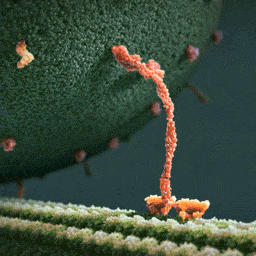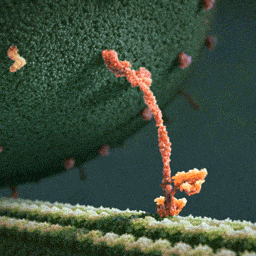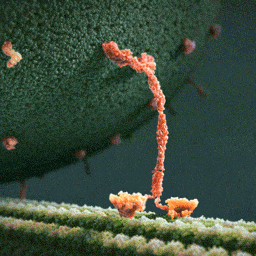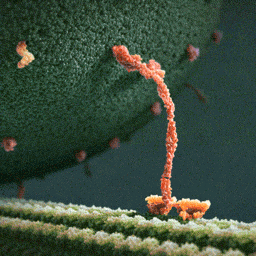
Myosin, kinesin, and dynein are important proteins governing internal transport. Myosin attached to organelles associates with actin microfilaments to enable the continuous flow of cytoplasm called cytoplasmic streaming.
Kinesins and dynein enable the movement of organelles along microtubules. They attach and move along microtubules. Most kinesins transport organelles from the center towards the periphery of the cell, anterograde transport. Dynein, and a few types of kinesins transport towards the cell center, retrograde transport.
(via thelifeofapremed)

(Image caption: A new technique called magnified analysis of proteome (MAP), developed at MIT, allows researchers to peer at molecules within cells or take a wider view of the long-range connections between neurons. Credit: Courtesy of the researchers)
Imaging the brain at multiple size scales
MIT researchers have developed a new technique for imaging brain tissue at multiple scales, allowing them to peer at molecules within cells or take a wider view of the long-range connections between neurons.
This technique, known as magnified analysis of proteome (MAP), should help scientists in their ongoing efforts to chart the connectivity and functions of neurons in the human brain, says Kwanghun Chung, the Samuel A. Goldblith Assistant Professor in the Departments of Chemical Engineering and Brain and Cognitive Sciences, and a member of MIT’s Institute for Medical Engineering and Science (IMES) and Picower Institute for Learning and Memory.
“We use a chemical process to make the whole brain size-adjustable, while preserving pretty much everything. We preserve the proteome (the collection of proteins found in a biological sample), we preserve nanoscopic details, and we also preserve brain-wide connectivity,” says Chung, the senior author of a paper describing the method in the July 25 issue of Nature Biotechnology.
The researchers also showed that the technique is applicable to other organs such as the heart, lungs, liver, and kidneys.
The paper’s lead authors are postdoc Taeyun Ku, graduate student Justin Swaney, and visiting scholar Jeong-Yoon Park.
Multiscale imaging
The new MAP technique builds on a tissue transformation method known as CLARITY, which Chung developed as a postdoc at Stanford University. CLARITY preserves cells and molecules in brain tissue and makes them transparent so the molecules inside the cell can be imaged in 3-D. In the new study, Chung sought a way to image the brain at multiple scales, within the same tissue sample.
“There is no effective technology that allows you to obtain this multilevel detail, from brain region connectivity all the way down to subcellular details, plus molecular information,” he says.
To achieve that, the researchers developed a method to reversibly expand tissue samples in a way that preserves nearly all of the proteins within the cells. Those proteins can then be labeled with fluorescent molecules and imaged.
The technique relies on flooding the brain tissue with acrylamide polymers, which can form a dense gel. In this case, the gel is 10 times denser than the one used for the CLARITY technique, which gives the sample much more stability. This stability allows the researchers to denature and dissociate the proteins inside the cells without destroying the structural integrity of the tissue sample.
Before denaturing the proteins, the researchers attach them to the gel using formaldehyde, as Chung did in the CLARITY method. Once the proteins are attached and denatured, the gel expands the tissue sample to four or five times its original size.
“It is reversible and you can do it many times,” Chung says. “You can then use off-the-shelf molecular markers like antibodies to label and visualize the distribution of all these preserved biomolecules.”
There are hundreds of thousands of commercially available antibodies that can be used to fluorescently tag specific proteins. In this study, the researchers imaged neuronal structures such as axons and synapses by labeling proteins found in those structures, and they also labeled proteins that allow them to distinguish neurons from glial cells.
“We can use these antibodies to visualize any target structures or molecules,” Chung says. “We can visualize different neuron types and their projections to see their connectivity. We can also visualize signaling molecules or functionally important proteins.”
High resolution
Once the tissue is expanded, the researchers can use any of several common microscopes to obtain images with a resolution as high as 60 nanometers — much better than the usual 200 to 250-nanometer limit of light microscopes, which are constrained by the wavelength of visible light. The researchers also demonstrated that this approach works with relatively large tissue samples, up to 2 millimeters thick.
“This is, as far as I know, the first demonstration of super-resolution proteomic imaging of millimeter-scale samples,” Chung says.
“This is an exciting advance for brain mapping, a technique that reveals the molecular and connectional architecture of the brain with unprecedented detail,” says Sebastian Seung, a professor of computer science at the Princeton Neuroscience Institute, who was not involved in the research.
Currently, efforts to map the connections of the human brain rely on electron microscopy, but Chung and colleagues demonstrated that the higher-resolution MAP imaging technique can trace those connections more accurately.
Chung’s lab is now working on speeding up the imaging and the image processing, which is challenging because there is so much data generated from imaging the expanded tissue samples.
“It’s already easier than other techniques because the process is really simple and you can use off-the-shelf molecular markers, but we are trying to make it even simpler,” Chung says.

This Week in Chemistry: Preventing marble statue weathering, further progress towards hydrogen fusion, and more! Links: http://goo.gl/WeJRV5

Swarms of magnetic bacteria could be used to deliver drugs to tumors
Researchers funded in part by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) have recently shown that magnetic bacteria are a promising vehicle for more efficiently delivering tumor-fighting drugs. They reported their results in the August 2016 issue of Nature Nanotechnology.
Ouajdi Felfoul, Mahmood Mohammadi, Samira Taherkhani, Dominic de Lanauze, Yong Zhong Xu, Dumitru Loghin, Sherief Essa, Sylwia Jancik, Daniel Houle, Michel Lafleur, Louis Gaboury, Maryam Tabrizian, Neila Kaou, Michael Atkin, Té Vuong, Gerald Batist, Nicole Beauchemin, Danuta Radzioch, Sylvain Martel. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nature Nanotechnology, 2016; DOI: 10.1038/nnano.2016.137
Illustration showing magnetic bacteria delivering drugs to a tumor. Credit: NanoRobotics Laboratory, Polytechnique Montreal










Watch: Bill Nye uses science to defend women’s reproductive rights
Follow @the-future-now
DNA from the deep? Antikythera shipwreck yields ancient human bones
Death, when it came, was sudden and cruel. The individual, either a crew member or passenger, was trapped on board when the huge ship foundered. Dashed on the rocks, the vessel slid beneath the waves, tumbled down an undersea cliff, and swiftly became buried in sediment on the seabed.
Now, more than 2,000 years later, archaeologists have recovered the bones of the individual they now call Pamphilos. Thought to be a man in his late teens to early 20s, he was on the ship sailing from Asia Minor to Rome when disaster struck off the tiny Greek island of Antikythera between Crete and the Peloponnese.
-
 techjum reblogged this · 4 years ago
techjum reblogged this · 4 years ago -
 kubrick06010 reblogged this · 7 years ago
kubrick06010 reblogged this · 7 years ago -
 whalesandcraftbeers reblogged this · 8 years ago
whalesandcraftbeers reblogged this · 8 years ago -
 grungletungus liked this · 8 years ago
grungletungus liked this · 8 years ago -
 starhasarrived reblogged this · 8 years ago
starhasarrived reblogged this · 8 years ago -
 starhasarrived liked this · 8 years ago
starhasarrived liked this · 8 years ago -
 catsfurever reblogged this · 8 years ago
catsfurever reblogged this · 8 years ago -
 ammosmb reblogged this · 8 years ago
ammosmb reblogged this · 8 years ago -
 the-nomadic-writer reblogged this · 8 years ago
the-nomadic-writer reblogged this · 8 years ago -
 doctorcrowd reblogged this · 8 years ago
doctorcrowd reblogged this · 8 years ago -
 firbalicious liked this · 8 years ago
firbalicious liked this · 8 years ago -
 peri-peri-tofu-blog liked this · 8 years ago
peri-peri-tofu-blog liked this · 8 years ago -
 fluidity reblogged this · 8 years ago
fluidity reblogged this · 8 years ago -
 mysteriesofcake reblogged this · 8 years ago
mysteriesofcake reblogged this · 8 years ago -
 hobbescreek reblogged this · 8 years ago
hobbescreek reblogged this · 8 years ago -
 theshinymew liked this · 8 years ago
theshinymew liked this · 8 years ago -
 sixthrangerknight reblogged this · 8 years ago
sixthrangerknight reblogged this · 8 years ago -
 aerstic-spire reblogged this · 8 years ago
aerstic-spire reblogged this · 8 years ago -
 gentianablue reblogged this · 8 years ago
gentianablue reblogged this · 8 years ago -
 daenigma liked this · 8 years ago
daenigma liked this · 8 years ago -
 mllescarlet reblogged this · 8 years ago
mllescarlet reblogged this · 8 years ago -
 narse-tantalus reblogged this · 8 years ago
narse-tantalus reblogged this · 8 years ago -
 narse-tantalus liked this · 8 years ago
narse-tantalus liked this · 8 years ago -
 devourer-of-acetone reblogged this · 8 years ago
devourer-of-acetone reblogged this · 8 years ago -
 mr5ivethou5and reblogged this · 8 years ago
mr5ivethou5and reblogged this · 8 years ago -
 starhasarrived reblogged this · 8 years ago
starhasarrived reblogged this · 8 years ago -
 fanofwings reblogged this · 8 years ago
fanofwings reblogged this · 8 years ago -
 gentianablue liked this · 8 years ago
gentianablue liked this · 8 years ago -
 juhakoskinen-blog liked this · 8 years ago
juhakoskinen-blog liked this · 8 years ago -
 60000fps liked this · 8 years ago
60000fps liked this · 8 years ago -
 bobshush reblogged this · 8 years ago
bobshush reblogged this · 8 years ago -
 ammosmb liked this · 8 years ago
ammosmb liked this · 8 years ago -
 insert-lesbian-related-pun reblogged this · 8 years ago
insert-lesbian-related-pun reblogged this · 8 years ago -
 adrianitacl-blog liked this · 8 years ago
adrianitacl-blog liked this · 8 years ago -
 besselfunctions reblogged this · 8 years ago
besselfunctions reblogged this · 8 years ago -
 communistcanuck liked this · 8 years ago
communistcanuck liked this · 8 years ago -
 allthechildren liked this · 8 years ago
allthechildren liked this · 8 years ago -
 set-saiil liked this · 8 years ago
set-saiil liked this · 8 years ago -
 deathcomes4u reblogged this · 8 years ago
deathcomes4u reblogged this · 8 years ago -
 leighta reblogged this · 8 years ago
leighta reblogged this · 8 years ago -
 the-telescope-times liked this · 8 years ago
the-telescope-times liked this · 8 years ago -
 the-telescope-times reblogged this · 8 years ago
the-telescope-times reblogged this · 8 years ago -
 udaitaxim liked this · 8 years ago
udaitaxim liked this · 8 years ago -
 herrothere liked this · 8 years ago
herrothere liked this · 8 years ago
A pharmacist and a little science sideblog. "Knowledge belongs to humanity, and is the torch which illuminates the world." - Louis Pasteur
215 posts